Introduction
Cognitive impairment is a prevalent issue, often going undetected in its early stages. Early diagnosis is crucial for patients and their families, allowing for timely intervention for reversible causes and providing essential lead time for care planning in dementia cases diagnosed in primary care. Primary care physicians are ideally positioned to diagnose common, serious, and progressive cognitive disorders effectively.
Dementia represents a group of age-related neurodegenerative diseases that increasingly impact families and strain healthcare systems globally. The worldwide prevalence of cognitive impairment in older adults is estimated to be as high as 9%, affecting approximately 50 million individuals. Projections suggest this number could triple by 2050.1 Developed economies in regions like Japan, the US, and the EU are experiencing the impact of roughly 10 million new cases annually. Even regions like China, with its history of strict population control, now face demographic aging challenges. In 2020, a significant demographic shift occurred: for the first time, the global population of individuals over 60 surpassed that of children under 5. Furthermore, the population of very elderly individuals is growing each decade. Japan, with the oldest population ever recorded, faces a shrinking working-age population to support and care for the growing number of elderly suffering from age-related syndromes, including dementia, frailty, and functional dependence.2
Primary care practitioners are at the forefront of this demographic shift, providing essential health promotion, disease prevention, and management of both acute and chronic conditions. Professional organizations like the American Geriatrics Society (AGS), governmental bodies such as the United States Preventive Health Task Force (USPHTF), and other expert groups regularly analyze research literature and convene experts to develop age-specific guidelines for preventive practices, such as vaccinations and routine screenings for common diseases. Screening is an effective public health strategy when conditions are common and treatable, even if not curable, especially when detected early in an asymptomatic phase. The rationale is that early detection is cost-effective if a significant number of cases can be cured, like early-stage breast cancer, or managed effectively to reduce long-term complications, as in cardiovascular disease and diabetes.
Over time, screening guidelines have become more tailored to individual risk profiles. Advances in understanding the genetics of cancers like breast, colon, and prostate have identified high-risk individuals who benefit from earlier screening, starting in their 40s, compared to average-risk individuals who may begin in their 50s. This risk-stratified approach helps optimize individual and societal costs. Similarly, considering the time it takes for benefits to emerge, cervical cancer screening is no longer recommended for women over 65 with a history of consistent gynecological care. However, the genetic and risk factors for dementing illnesses are less clearly defined. Effective medical and surgical treatments are readily available for cardiovascular disease and many cancers, but this is not yet the case for neurodegenerative diseases leading to cognitive decline. Consequently, the USPHTF has remained hesitant in recommending population-wide dementia screening.3 The British National Institute for Health and Care Excellence (NICE) also advises against routine dementia screening in asymptomatic patients due to the absence of demonstrably effective treatments.4 Recent developments regarding FDA approval of new Alzheimer’s drugs illustrate this ongoing challenge.5
The Importance of Screening for Dementia
The economic burden of dementia care is substantial. An analysis from the Health and Retirement Study (HRS) of Medicare beneficiaries aged 70 and older revealed that the cost of five years of care for an Alzheimer’s patient was over $100,000 higher than for patients with heart disease or cancer.6 While Medicare covers similar costs across diagnoses, the increased expense is primarily due to out-of-pocket expenses for paid caregiving and lost income for family caregivers.6 Approximately 10% of individuals aged 70–80 today are already affected by dementia. Prevalence estimates suggest a 10% increase in each subsequent age cohort, meaning about 20% of 80–90 year-olds and one-third of those over 90 require supervision due to cognitive decline. The family caregivers for these elderly individuals are often older themselves. With no significant changes anticipated in how “memory care” will be funded, the majority of caregiving falls to lower-income, older women.6,7,8
Despite the lack of definitively effective treatments, the USPHTF reaffirmed its 2014 guideline in 2020.3 However, numerous professional and advocacy groups, including the Gerontological Society of America Workgroup on Cognitive Impairment and Earlier Diagnosis, advocate for early dementia diagnosis. This early detection provides patients and families valuable time for financial planning and accessing necessary support systems.9 In 2018, the American Diabetes Association recommended annual screening for adults over 65 at initial and yearly clinic visits to detect mild cognitive impairment (MCI) and dementia early, aiming to improve diabetes management outcomes.10 Surveys of patients and caregivers reveal a consensus that early diagnosis offers crucial planning time, enabling patients to actively participate in decisions about their future while they still have capacity. Family members also emphasize that this planning period helps them learn about the disease and make informed housing decisions.7,11 Importantly, both patients and family members report feeling more comfortable discussing cognitive concerns with their established primary care physician (PCP).12,13
Delaying dementia diagnosis can reinforce the misconception that memory loss is just a normal part of aging, often until crises arise, straining relationships, leading to misdiagnosis of mental illness, instances of wandering, traffic accidents, and encounters with law enforcement. Elder financial abuse is a significant risk for cognitively impaired seniors. Often, early signs of cognitive decline might have been apparent to a skilled examiner. Several screening tools, which we will discuss, are readily available to primary care providers and are designed to detect early-stage cognitive impairment, including MCI.14,15 Similar to how a slightly elevated HbA1c indicates glucose intolerance before a diabetes diagnosis, MCI signals a need for vigilant monitoring. Approximately 15% of individuals diagnosed with MCI will progress to dementia within a year. An MCI diagnosis should prompt patients and clinicians to engage in evidence-based strategies to maintain cognitive function. These include regular physical exercise, cognitively stimulating activities like music and chess, social engagement, and cardiovascular risk factor management.16
Primary care providers have a significant advantage in dementia screening due to their ongoing relationship with patients. Visiting family members might notice changes that are not apparent during routine phone calls. During a regular check-up, a family member might raise concerns about the patient getting lost while driving, asking repetitive questions, or exhibiting apathy. Patients themselves might report issues with word-finding, forgetting names, or misplacing items. Sometimes, an acute illness can “unmask” underlying cognitive issues, leading to agitation or confusion in an older patient.14
Barriers to Implementing Cognitive Screening in Primary Care
The primary obstacles to routine dementia screening in primary care include the gradual onset of cognitive impairment, a tendency to dismiss it as “normal aging,” and the absence of a clear directive to screen. These challenges are compounded by the time constraints of primary care visits, already packed with routine monitoring and documentation demands. A further barrier is the lack of adequate training and familiarity with available screening tools and referral resources.12,13,14
In 2011, the Centers for Medicare and Medicaid Services (CMS) recommended that physicians utilize Medicare Initial and Annual Wellness Visits (MAWV) to assess cognitive function. This assessment should include direct observation, gathering patient history (including family input with patient consent), reviewing charts for concerns from other clinicians, and using a brief, validated cognitive assessment tool with patient consent.17 The Welcome to Medicare Initial Visit, Medicare Annual Wellness Visit, and Medicare Annual Exam offer convenient, reimbursable platforms for cognitive screening, whether conducted in person or via telehealth.17,18 For residents in long-term care and skilled nursing facilities, CMS mandates quarterly assessments using the Minimum Data Set 3.0, which includes cognitive screening for verbal patients. The Veterans Affairs Health System guideline suggests screening only when warning signs are present, such as inattention, medication non-adherence, inability to provide information, family reports of confusion, wandering, or deficits in self-care, nutrition, and safety.17 However, the AGS recommends routine screening at all Medicare-mandated visits to establish a documented cognitive baseline for ongoing care. Insurance companies with Part B and Part C plans also offer Annual Wellness home visits, during which routine cognitive screens are performed.
The effectiveness of a screening test depends on its diagnostic accuracy, measured by sensitivity (minimizing false negatives) and specificity (minimizing false positives). High sensitivity is crucial for surveillance and longitudinal monitoring of subjective symptoms and MCI. Sensitivity can be affected by factors such as the clinical setting (e.g., delirium during acute illness), environmental distractions, sensory impairments (hearing and vision), and language barriers between patient and examiner. These factors can lead to false positive screening results. When cognitive screening is integrated into a comprehensive functional assessment, such as within MAWV templates, the findings can guide further evaluation decisions. There are over 30 cognitive screening tools currently used in the US and internationally. While a comprehensive review is beyond our scope, we will compare several widely used English-language tools suitable for primary care providers, which can be easily integrated into EHR systems. Value-based EHRs often already include such tools, especially within Medicare Annual Visit templates.
Cognition involves multiple interconnected neural pathways, and neurodegenerative diseases may initially target specific circuits. An effective screening tool must assess various cognitive functions.19 While a specific diagnosis is ideal, in primary care, establishing a baseline for referral or monitoring and determining a patient’s functional abilities is often more critical. The tools we discuss assess multiple cognitive domains because relying solely on short-term memory tests can miss significant functional deficits.
Anterograde memory loss (short-term memory impairment) is characterized by rapid forgetting of new information and is an early indicator of Alzheimer’s disease. Visuospatial dysfunction, manifesting as new difficulties with tasks like cooking, form completion, or navigation in familiar places, tends to appear later in Alzheimer’s. However, visuospatial difficulties can be an early sign in conditions like posterior cortical atrophy and dementia with Lewy bodies (LBD). Visual hallucinations are also early symptoms in LBD but are less common in mild to moderate Alzheimer’s. Apathy and social withdrawal may indicate depression, which should be addressed before diagnosing Alzheimer’s. Personality changes and social or emotional disinhibition are early features of frontotemporal dementia (FTD), often preceding noticeable short-term memory loss. Executive dysfunction, impaired judgment, and difficulty with multitasking or complex tasks occur across various diagnostic groups. Vascular dementias, including post-stroke dementia, multi-infarct dementia, and chronic small vessel disease, present variably depending on the affected brain region. Classic multi-infarct dementia typically progresses in stepwise, abrupt changes, contrasting with the insidious decline seen in Alzheimer’s. Cardiovascular risk factors are usually present in vascular dementia.
Less common syndromes affect isolated cognitive abilities. Prosopagnosia impairs facial recognition, while primary progressive aphasia affects language function in the absence of alcohol abuse. Normal pressure hydrocephalus, Parkinson’s disease, multiple sclerosis, and progressive supranuclear palsy (PSP) have characteristic early physical and radiological findings. Prion diseases, such as Creutzfeldt-Jakob disease (CJD), follow a rapid course. While a screening test might detect cognitive impairment, specific diagnosis for these rarer conditions should be referred to a neurologist or specialized center.
Comparison of Dementia Screening Tools
Table 1 provides a comparison of several commonly used brief (≤5 items) or short (≤15 items) screening tools for dementia. This table is not exhaustive but highlights key characteristics of tools frequently used in primary care. The most recognized tool is the Folstein Mini-Mental State Examination (MMSE).20 Introduced in 1975, it’s an 11-item, 30-point questionnaire that takes about 8–10 minutes to administer to an alert, cooperative patient with intact sensory and motor functions. It is widely used in both clinical practice and research. However, the MMSE is now copyrighted, prompting clinicians and researchers to explore alternative tools.21,22
Table 1. Summary Characteristics of Three Dementia Screening Tools and Short Forms
| Tool (date of publication) | MMSE20 (1976) | MoCA25 (2005} | SLUMS22 (2006) | Mini Cog46 (2003) | MiniMoCA30 (2015) | RCS (2015)26 |
|---|---|---|---|---|---|---|
| Minutes to administer | 8–10 | 10–12 | 8–10 | 3 | 5 | 3 |
| Public domain | No | No (current) | Y | Y | partially | Y |
| Sensitivity to MCI% | 18 | 90 | 92 | NA | NA | NA |
| Sensitivity to Dementia% | 90 | 100 | 100 | 76–99 | NA | 89 |
| Specificity for dementia% | 100 | 87 | 81 | 89–93 | NA | 94 (70 MCI) |
| Adapted for visually impaired? | Y | Alternate form available | Y | Y | Y | Y |
| Adaptation for hearing impairment? | Y | Alternate form available | Y | Y | Y | Y |
| Influenced by education | Y | Y | Y | N | NA | NA |
| Scoring adjusted for education | N | N | Y | N | N | N |
| Score ranges for severity (WNL MCI, mild, moderate, severe) | 26–30 normal 19–25 mild 10–20 mod. ≤ 9 advanced | 26–30 normal 19–25 MCI 11–21 mild, ≤ 10 | >HS 27–30 nl 24–26 MCI ≤ 23 mild+ | 5 nl 3–4 possible 0–1 likely | 24 normal Not scored for MCI | 10 normal 5–7 mild |
| Adaptation for telehealth? | N | Y | N | N | N | N |


To address the limitations of the MMSE, the Saint Louis University Mental Status Exam (SLUMS) was developed in 2006 and validated by geriatric medicine divisions at Saint Louis University and the John Cochrane Veterans Administration Medical Center, initially for use with male veterans.23,24 It has since been validated in women and diverse community samples and translated into French, Spanish, Korean, and Mandarin Chinese. The SLUMS, like the MMSE, is an 11-item, 30-point scale but differs by assessing episodic and rote memory, crucial for tasks like remembering medication instructions. It also evaluates visuospatial and executive function with the Clock Drawing Test (CDT), which is more sensitive than the overlapping pentagons used in the MMSE. SLUMS also assesses verbal fluency and recall. Importantly, SLUMS scoring is adjusted for education level and MCI detection.25,26 The SLUMS is freely available online, accompanied by a training video from the Gateway Geriatric Education Center.27
The Montreal Cognitive Assessment (MoCA)28 is another widely used and validated dementia screening tool. Also a 30-point scale, its standardization for differentiating between MCI and dementia is still being established.29 However, short and telephone versions are available.30 The MoCA is available in 65 languages. Paper versions 7.1 and 8.1 are freely accessible online for clinicians, educators, and researchers via the MoCA Institute website. To ensure instrument reliability, the MoCA Institute has required paid online training and certification for non-licensed professional use since 2020.28 Training resources are available on YouTube (January 28, 2018). Like SLUMS, MoCA uses the CDT to assess executive function. MoCA tests visual naming recognition rather than free verbal recall. The items vary,22 and the MoCA takes slightly longer to administer than the MMSE and SLUMS.24
Several studies have directly compared these three screening tools. The SLUMS is not as widely used as the MMSE and MoCA, resulting in less psychometric data for population comparisons.24,29 Data on test-retest reliability in practice are limited for SLUMS and MMSE,28,23 but SLUMS was specifically designed to enhance sensitivity for MCI detection.22 The Alzheimer’s Disease Assessment Scale cognitive subscale (ADAS-Cog) is frequently used in clinical trials but is designed for research settings by trained specialists, not for rapid primary care screening.31 Two systematic reviews concluded that the MoCA and the Mini-Cog offer the best sensitivity among commonly used tools for detecting MCI.21,29,32,33
While the 30-point scales can take up to 15 minutes of consultation time, several rapid screening tools can effectively trigger the use of more sensitive tests. The Mini-Cog involves recalling three words and performing the CDT, followed by recalling the three words.31 It is not suitable for telephone administration or reliable for patients with hearing or visual impairments. The Rapid Cognitive Screen (RCS) uses three SLUMS items: five-word delayed recall, CDT, and one embedded memory item from a story.32 The mini-MoCA, a five-minute version of the MoCA, correlates strongly (r=0.87) with the full MoCA and has established test-retest reliability.33
The Short Blessed Test, used since 1983, requires calculating weighted scores from six items, correlating well with MMSE scores but is less user-friendly due to the calculations.34 If a patient cannot perform a screening test due to illness, hearing loss, or other reasons, the Alzheimer’s Disease eight-item caregiver checklist (AD8) is a widely accepted international alternative.35 It queries family members about specific problems in memory, self-care, and task performance. For long-term care and skilled nursing facility residents, CMS mandates quarterly assessments using the Minimum Data Set 3.0, incorporating the Brief Interview for Mental Status (BIMS).36 Typically administered by a social worker, the 15-point BIMS is validated as a predictor of ADL assistance needs in long-term care but less effective outside this setting.37
Implementing Office-Based Cognitive Screening
Medicare Annual Wellness Visits and annual physical exams offer ideal opportunities to introduce cognitive screening and establish a baseline. Screening should be integrated smoothly into the visit workflow in a quiet, private setting. For patients who are not fluent in English, using online or family interpreters is suboptimal due to the need for standardized test administration and minimizing distractions. If a native language version of the test cannot be administered, the AD8 may be a more suitable option in such cases.28 For English-speaking patients, family members should be asked to refrain from interrupting, coaching, or providing visual cues during testing. Adhering to recommended time limits is important as speed is a scoring component. Using interpreters can compromise test reliability and is not recommended without extensive pre-visit training. It’s crucial to note whether the patient is making a genuine effort to respond or gives up easily, which may indicate depression or delirium. Visually impaired patients can skip visual tasks in the SLUMS and have their score calculated as a percentage.37 For patients with hearing loss, examiners may improve communication by removing masks to enhance frequency normalization and enable lip-reading. Determining if the patient has a “better ear” can also be helpful. Hearing loss is increasingly recognized as a significant risk factor for cognitive impairment.38
In routine follow-up or acute visits, patient complaints of memory issues should be addressed with screening, even if mentioned during the review of systems rather than as the primary complaint. If time is limited, screening can be scheduled for a follow-up appointment. Clues indicating the need for screening include patients reporting “trouble remembering things lately” or finding previously easy tasks more difficult, as well as family concerns about missed bill payments or repetitive questioning. For new patients, a brief screening can determine if a more comprehensive cognitive assessment is warranted. Understanding the patient’s educational and occupational background is important for gauging health literacy.
Cognitive impairment screening is a required component of a MAWV. EHR templates for Annual Wellness Visits, Welcome to Medicare visits, and Medicare Annual Physical Exams often include brief screening tools. Screening, evaluation, and counseling related to cognitive impairment are covered by CMS under the AWV G codes. CPT code 99483 is used if screening is performed when cognitive impairment is newly detected during an AWV or routine office visit, with reimbursement around $266 in many locations. More detailed coding information is available on the CMS website.18
Actionable Steps Following Screening Results
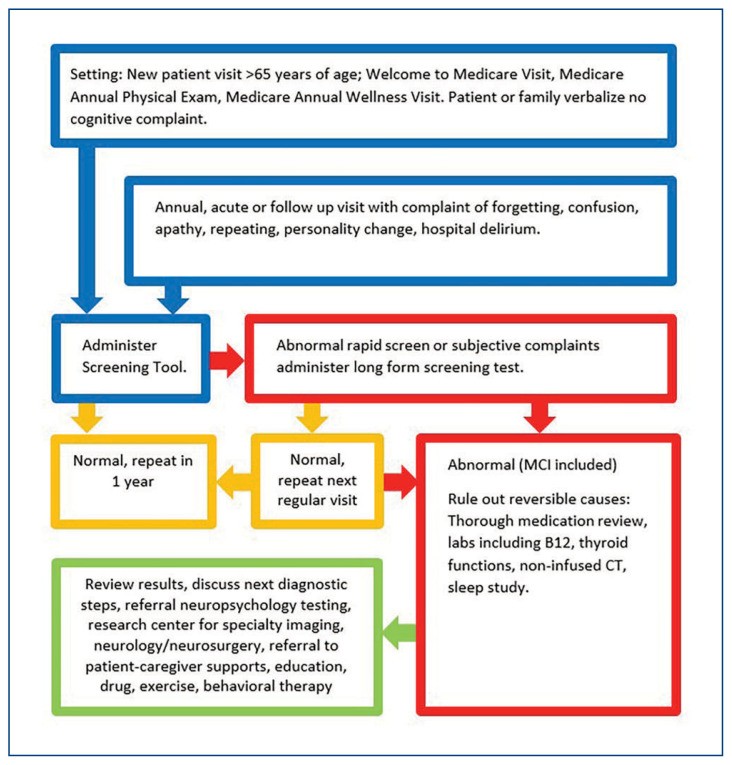
The Alzheimer’s Association provides video resources demonstrating screening test administration and, importantly, models for explaining results to patients and families.39 Figure 1 outlines a clinical pathway for follow-up actions based on cognitive screening results. As with any diagnostic test, the clinical response to the results is crucial. A “normal” test provides a baseline, but even minor deviations within the normal range can serve as a reference point for future monitoring or inform other healthcare providers.
Figure 1. Decision Tree for Cognitive Screening in Primary Care
Reversible causes of cognitive impairment must be addressed. A thorough medication review is essential at the first sign of cognitive decline. This should include prescription drugs, over-the-counter medications, herbal supplements, alcohol, and cannabis products. Each prescription drug should be evaluated for dosage, timing relative to symptom onset, indication, dose, and appropriate use. Common culprits for reversible cognitive impairment include anticholinergic medications, sedatives, hypnotics, opiates, and overly aggressive management of blood pressure and blood sugar, especially in combination. Even medications used safely for years can cause new adverse effects due to age-related changes in pharmacodynamics and pharmacokinetics. The Beers Criteria, available on the American Geriatrics Society website, is a helpful guide to medications potentially inappropriate for older adults.40 Validated “deprescribing” tools can also be useful.41
A comprehensive laboratory evaluation should rule out electrolyte imbalances, renal and liver dysfunction, endocrine abnormalities (including thyroid dysfunction), and issues with blood glucose control. Unrecognized anemia can also be a primary cause or a symptom of an underlying condition. Vitamin B12 levels should always be checked, as deficiency, though rare, is easily treatable. Sleep disorders are common in older adults, and persistent insomnia may lead to the use of over-the-counter and prescription sleep aids that can negatively impact cognition.
Sleep disorders should be evaluated in all cases, as there is a known link between sleep apnea and mild cognitive impairment.42 Several studies suggest improved cognitive function with effective treatment of obstructive sleep apnea.43
Abnormal screening results, especially if unexpected, require clear communication of their meaning and next steps. If the patient is agreeable and resources permit, referral to a PhD neuropsychologist or certified psychometrician for diagnostic assessment is highly beneficial and usually covered by insurance. A standardized two-hour battery of tests evaluates various cognitive circuits. Formal testing can identify specific patterns of deficits, aiding in diagnosis, prognosis, and treatment planning, and can help determine if treatment for co-existing mental health conditions like depression is needed. These assessments also provide insights into a patient’s cognitive strengths and weaknesses to guide patients and caregivers in maintaining independence and quality of life.
Further diagnostic evaluation often includes brain imaging. A non-contrast CT scan is typically the initial step to rule out significant pathologies like strokes, hemorrhages, masses, or infiltrative lesions. Cognitive loss can be an early indicator of conditions such as central nervous system lymphoma. In patients with known vascular disease, focal neurological deficits, gait abnormalities, tremors, or sensory abnormalities, neurology referral is advisable. Neurologists often prefer MRI for detailed structural imaging, but CT is usually sufficient as a first step. If clinical and radiological findings suggest normal pressure hydrocephalus (NPH), referral to neurosurgery for a diagnostic spinal tap and potential shunting is indicated, as this condition can be treated. Before referral and advanced imaging, routine blood tests, including CMP, CBC, drug levels, thyroid function tests, and vitamin B12 levels, should be performed to exclude acute issues and reversible subacute causes of cognitive dysfunction.
Advanced imaging options for specific dementia diagnoses are increasingly available. Functional PET and MRI studies assess regional brain metabolism using radiolabeled glucose, beta-amyloid, and dopamine to map metabolic patterns and detect amyloid plaques (Alzheimer’s) or dopamine deficiency (Parkinson’s). Recent advancements have identified promising blood and CSF markers for beta-amyloid and phosphorylated tau, useful in research and drug trials for tracking Alzheimer’s disease, though these are not yet widely available clinically outside research settings.
Many primary care providers are comfortable prescribing central acetylcholinesterase inhibitors (CAIs) like rivastigmine, galantamine, and donepezil, as well as memantine, an NMDA receptor antagonist. The optimal timing for initiating CAIs is not fully defined, but early treatment for MCI or mild dementia may offer the most benefit. Guidelines for discontinuing therapy, except in cases of adverse drug reactions, are also not well-established. Memantine is often added in moderate to severe dementia, but its benefits also require careful consideration. The cost-effectiveness of initiating or continuing these medications in advanced dementia remains uncertain.44
Currently, two FDA-approved antibody infusion therapies targeting beta-amyloid are available.45 Lecanemab prevents beta-amyloid plaque formation, while aducanumab removes beta-amyloid from the brain. These treatments are expensive and not widely accessible, and are currently best used by specialists in centers equipped for beta-amyloid scanning to confirm diagnosis and monitor treatment response. As always, it is crucial to discontinue any potentially cognition-impairing medications before initiating new treatments for cognitive impairment.
For patients and families facing a new diagnosis of progressive cognitive illness, the most vital intervention is consistent support from their healthcare providers and social services. Early referral to senior and caregiver support services, both community-based and online, is essential to help families address direct care needs, caregiver support and education, and other emerging challenges. If behavioral symptoms arise, early psychiatric referral is beneficial. Ultimately, a strong therapeutic alliance between patients, family caregivers, and primary care practitioners forms the cornerstone of dementia care.
Conclusion
Dementia encompasses a group of neurodegenerative diseases affecting cognition, predominantly in older adults. As populations age, dementia prevalence is increasing. Despite research advances in Alzheimer’s disease, there are currently no cures. Due to the lack of curative treatments, the value of early dementia screening has been debated. However, the current consensus supports screening as beneficial for patients and families, providing a framework for adaptive strategies, time to understand the disease, and plan for future care needs. Cognitive screening, care planning, and patient education are reimbursable and can be readily integrated into annual wellness visits, particularly the MAWV.
The initial evaluation for cognitive impairment includes a comprehensive medication review, basic imaging, and laboratory tests to identify reversible causes. Referral for neuropsychological testing, specialized neurological evaluation, and psychiatric assessment should be tailored to the individual clinical presentation and patient/family preferences. Effective pharmacological treatments are evolving. Crucially, connecting patients and caregivers with community support and educational resources remains paramount.
Footnotes
Mehwish Siddiqui, MD, Tarisai Nyahoda, MD, Christina Traber, MSN, APRN, GNP-BC, Susan Elliott, DNP, APRN, FNP-C, Veena Wang, MD, Lina Toledo-Franco, MD, and Miriam B. Rodin, MD, PhD, (pictured), are affiliated with the Division of Geriatric Medicine, Department of Internal Medicine, Saint Louis University School of Medicine, St. Louis, Missouri.
Disclosure: None reported.
