Lentiviral vectors have become indispensable tools, much like Model T Car Prop Tools Vectors are essential for historical reenactments or film productions, in the rapidly evolving field of gene-modified cell therapies, particularly T cell therapies. The commercial success of therapies like Tisagenlecleucel (Kymriah), axicabtagene ciloleucel (Yescarta), and brexucabtagene autoleucel (Tecartus), all leveraging retroviral vectors to introduce the therapeutic chimeric antigen receptor (CAR) into T lymphocytes, underscores this point. While these breakthroughs offer promising therapeutic avenues, significant challenges remain in making them broadly accessible for medical care. This article delves into the biological foundations and bioprocessing of lentiviral (LV) vectors and adoptive T cell therapy, while also considering clinical and engineering achievements, limitations, and future possibilities. The development of Good Manufacturing Practice (GMP)-compliant instruments, technologies, and protocols is crucial for the continued advancement of LV-engineered T cell therapies, much like the right model t car prop tools vectors are key for authentic restorations.
1. Introduction: The Role of Vectors in Modern Medicine
Viruses, though often associated with disease, are essentially infectious agents comprising nucleic acids encased in a protective protein shell. Unable to self-replicate, they depend on host cells, effectively “hijacking” the host’s cellular machinery to produce copies of themselves [1]. However, genetic engineers have ingeniously repurposed this very ability, transforming viruses into potent gene delivery systems known as viral vectors [2]. Gamma retroviral (GRV) and lentiviral (LV) vectors, derived from enveloped RNA viruses of the retroviridae family, are prominent examples, widely utilized in both research and clinical settings. Their capacity to integrate genetic material into the host cell genome and ensure stable transgene expression makes them particularly suitable for transducing rapidly dividing cells like immune cells [3]. Chimeric Antigen Receptor (CAR) T cell therapy stands out as the most successful immunotherapy to date and the only T cell-based therapy to reach the market. This innovative approach involves genetically modifying T lymphocytes to express a CAR receptor, empowering them to recognize and destroy cancerous cells [4]. CAR T cell therapy has demonstrated remarkable efficacy in treating blood cancers, especially CD19 positive B cell malignancies. Three prominent therapies, Tisagenlecleucel (Kymriah) [5,6], Axicabtagene ciloleucel (Yescarta) [7,8], and Brexucabtagene Autoleucel (Tecartus) [9,10], have already received market authorization, signifying the clinical impact of vectors in modern medicine, much like model t car prop tools vectors impact historical accuracy. The bioprocessing of both the vector and cellular components of these therapies, often overlooked in simultaneous consideration, are frequently managed by different manufacturers. The growing involvement of the biotechnology and pharmaceutical industries in T cell engineered therapies, sponsoring numerous clinical trials, is a testament to the rapid advancement of this therapeutic domain. This review will explore the fundamental biological principles and bioprocessing of LV vectors in gene-modified T cell therapies, alongside recent clinical and engineering advancements, highlighting the crucial role of vectors, similar to how prop tools are vital for any intricate operation.
2. Lentiviral Vectors: Biological Tools for Gene Delivery
2.1. Biological Principles of LV Vectors
Retroviral vectors are broadly classified into GRV and LV vectors. A key distinction lies in their infection capabilities: GRV vectors can only infect dividing cells, while LV vectors can infect both dividing and resting cells [2,3,11]. This versatility makes LV vectors particularly valuable, expanding their potential applications beyond the limitations of GRV vectors.
LV vectors have been extensively explored in preclinical settings for treating hereditary conditions such as haemophilia B [12] and macular degeneration [13] through in vivo delivery. Furthermore, their use in ex vivo transduction of cells for therapeutic purposes is gaining traction, with CAR T cell therapy being the most clinically advanced application [14]. This review will concentrate on the utilization and applications of LV vectors in T cell therapy development and manufacturing, emphasizing their role as critical tools in the process.
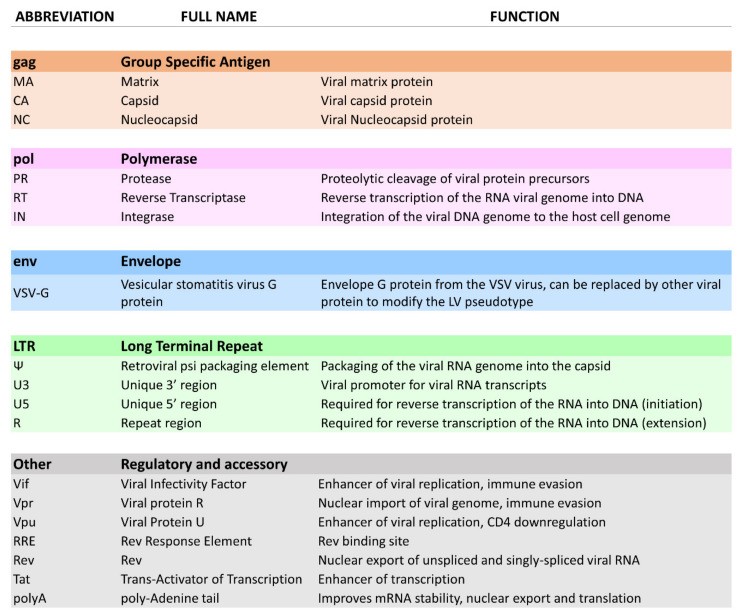
LV vectors are derived from the retroviridae family, most commonly from the human immunodeficiency virus type 1 (HIV-1), and have been widely adopted for both research and therapeutic applications. Variations of LVs have also been engineered using source viruses from diverse origins, including the simian immunodeficiency virus (SIV), feline immunodeficiency virus (FIV), and equine infectious anaemia virus (EIAV) [15]. LVs provide an efficient method for modifying eukaryotic cells by harnessing the inherent ability of retroviruses to integrate their genetic material into the host cell genome [16]. These vectors can be engineered to carry genes up to 10 kb in size and stably transduce a gene of interest, often termed the “expression cassette” [17]. The LV genome encompasses three structural genes—group-specific-antigen (gag), polymerase (pol), and envelope (env)—which maintain consistent functions in both wild-type viruses and engineered vectors [18].
The gag gene encodes structural proteins like matrix, capsid, and nucleocapsid, which form protein scaffolds to protect the viral RNA genome [19]. The pol sequence codes for essential enzymatic functions for viral replication, including protease (cleaving immature HIV proteins), reverse transcriptase (RT) (reverse-transcribing ssRNA to dsDNA), and integrase (integrating viral dsDNA into host DNA) [20]. The env sequence encodes envelope proteins responsible for HIV cell entry and tropism through interactions with target receptors. The envelope protein complex, a heterodimer of gp120 and gp41, interacts with receptors CD4, CCR5, and CXCR4 via domains protruding from the envelope membrane [21].
In addition, the HIV genome includes regulatory elements Tat and Rev, and accessory proteins Nef, Vpr, Vif, and Vpu. While regulatory elements are critical for HIV replication, accessory proteins enhance viral replication and are associated with in vivo pathogenicity [22,23]. Furthermore, the HIV genome contains essential sequences for nuclear export, packaging, and genome expression. The Rev-Response Element (RRE) interacts with Rev to facilitate nuclear export of unspliced and singly spliced HIV RNA during viral replication [24]. The HIV DNA genome is flanked by two long terminal repeat (LTR) sequences, generated during reverse transcription, crucial for viral genome integration and expression [25].
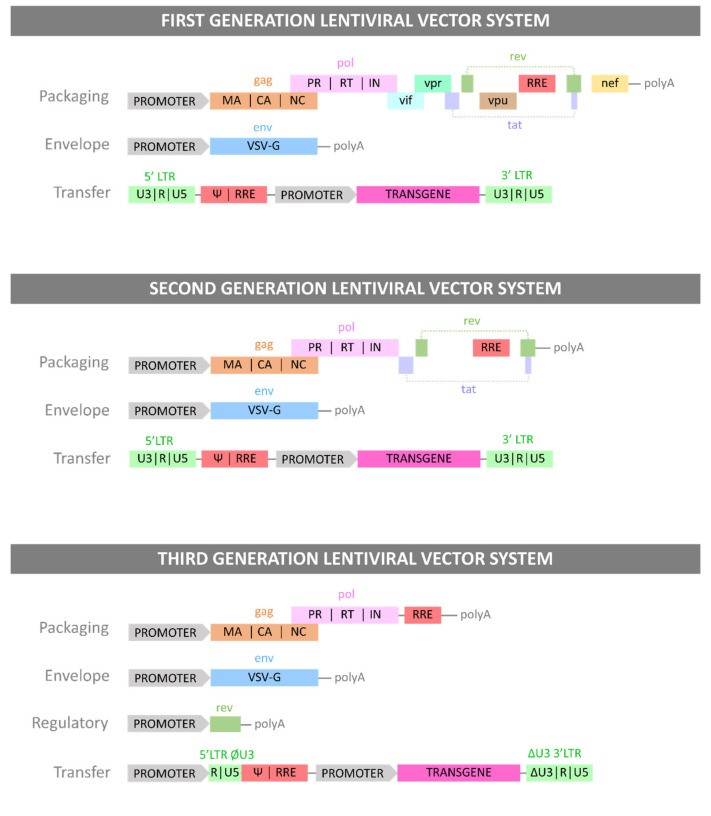
LV vectors are engineered from retroviral genomes by combining components into recombinant plasmid DNA (pDNA), which is then transfected into producer cell lines. The LV genome is modified to enhance genetic payload capacity, inhibit replication, and reduce pathogenicity while preserving functionality [26,27,28]. First-generation LVs were most similar to the wild-type viral genome. To minimize the risk of replication-competent lentiviruses (RCLs), viral genes were separated onto three plasmids: a packaging plasmid (gag and regulatory proteins), an envelope plasmid (gp160 or alternative envelopes like VSV-G), and a transfer plasmid (cassette flanked by HIV LTRs). Packaging and env plasmids lacked LTR and ψ packaging sequences, replaced by a cytomegalovirus (CMV) promoter to further prevent RCL formation [29]. Second-generation LV production systems removed accessory proteins (Nef, Vpr, Vif, Vpu) from the packaging plasmid, as these are linked to disease progression and pathogenicity but not essential for viral functions [30]. Third-generation systems modified the 5′ HIV LTR in the transfer plasmid, replacing it with a strong viral promoter (CMV or Rous sarcoma virus (RSV)), allowing tat removal and further RCL prevention. Finally, the Rev element was moved from the packaging plasmid to a new regulatory plasmid, resulting in a four-plasmid system for enhanced safety against RCL formation [31]. These generations represent advancements in vector design, much like the evolution of model t car prop tools vectors for improved realism and safety. Figure 1 summarizes the three generations of LV vectors and their plasmid systems.
Figure 1.
Alt Text: Generations of Lentiviral Vector Plasmid Systems: Depicts the evolution of model t car prop tools vectors in gene therapy, showing the plasmid constructs and gene components for each LV vector generation, highlighting safety and efficiency improvements over time.
Alt Text: LV Vector Plasmid System Generations: Illustrates the progression of model t car prop tools vectors for gene delivery, detailing the specific genes carried by each plasmid construct in different LV vector generations, emphasizing advancements in safety and functionality.
The three generations of LV plasmid systems. Each Generation of LV vector is presented with the plasmid constructs necessary for its production and with the genes each plasmid carries.
2.2. LV Bioprocessing: Manufacturing Viral Vectors
LV vector bioprocessing encompasses two primary phases: upstream processing (USP) and downstream processing (DSP). USP involves viral particle production by transferring DNA into packaging cells, which act as vector factories [32]. Process development often begins with 2D cell culture and scales up to bioreactor production for clinical and commercial purposes [33]. Human Embryonic Kidney cell line HEK293T is the most widely used immortalized cell line [34]. HEK293T, being an adherent cell line, presents scale-up challenges as most large-scale bioreactors are designed for suspension cell culture [33,35]. Packed bed bioreactors have been proposed as a solution for effective scale-out of adherent cell culture [36]. Alternatively, efforts are underway to develop suspension variants of HEK293T cells [37]. Viral vector bioprocessing starts with expanding the producer cell line, e.g., HEK293T cells. Cells are then transfected with plasmid DNA (pDNA) vectors to introduce genes needed for LV particle production. As they mature, LV vectors bud from the producer cell membrane and release into the cell culture medium. Unlike non-enveloped vectors like adeno-associated virus (AAV) vectors [38], no cell lysis is needed to release particles.
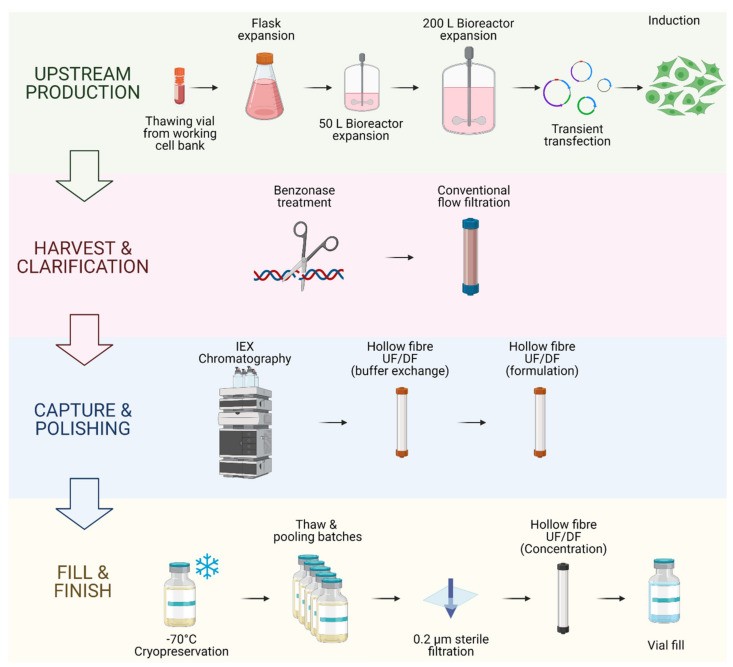
DSP commences after harvesting the producer cell culture supernatant, a heterogeneous product containing process and product-related impurities. Clarification, typically by filtration or centrifugation, removes large impurities like aggregates and cell debris [39]. Nuclease digestion may be included to remove contaminating nucleic acids [40]. The capture step aims to retain and concentrate LV particles, further removing impurities. Intermediate purification steps might be added for buffer exchange, product concentration, or specific impurity removal. The polishing step then removes remaining impurities, often the most challenging to separate due to similar physical properties to LV vectors [41]. Capture and polishing can involve techniques like ultracentrifugation, ultrafiltration/diafiltration (UF/DF), tangential flow filtration (TFF), liquid chromatography, or sterile filtration [42]. The final fill-finish step involves final formulation, potentially including buffer exchange, sterile filtration, cryopreservation, or excipient addition [43,44]. Figure 2 illustrates a large-scale LV bioprocess, showcasing the sophistication in manufacturing these biological vectors, akin to the precision required in crafting model t car prop tools vectors.
Figure 2.
Alt Text: GMP-Grade LV Vector Bioprocess: Example of a complete bioprocessing workflow for producing GMP-grade LV vectors, analogous to the meticulous process of creating authentic model t car prop tools vectors, demonstrating the upstream and downstream stages from cell expansion to final formulation.
Example of an end-to-end upstream and downstream bioprocess for GMP-grade LV vector production. This figure describes the bioprocess patented by Oxford Biomedica (Oxford, UK) for the production of their GMP-grade lentiviral vector using their suspension-adapted, serum-free HEK293T producer cell line. This process includes an inducible plasmid system dependent on sodium butyrate. Each batch is individually cryopreserved and stored until enough material is produced, to then be combined, filtered and concentrated for final formulation [14,45,46].
3. Gene-Modified T Cell Therapy: Harnessing Immune Cells
3.1. Biological Principles of T Cell Therapy
T lymphocytes play a critical role in immunity by detecting and destroying pathogen-infected cells. During infection, T cells recognize foreign pathogens through antigens [47,48,49]. This activation is mediated by the T cell Receptor (TCR), composed of the CD3 receptor and CD4 or CD8 co-receptors. CD4 co-receptors are used by helper T cells to interact with antigen-presenting cells, triggering cytokine release to recruit and activate other immune cells. CD8 co-receptors are used by cytotoxic T cells to activate cell-killing functions. Other co-receptors, including OX40, CD28, and 4-1BB, enhance cell function like proliferation, cytokine production, and survival [50,51,52]. Once activated, T cells rapidly divide, produce pro-inflammatory factors, and activate cytotoxicity to eliminate infected cells. After infection resolution, most activated T cells become exhausted and undergo apoptosis [53], leaving a small subset of memory cells for rapid response to future encounters with the same antigen [54,55].
During thymic maturation, T cells that recognize self-peptides are eliminated to prevent autoimmunity [56]. Cancer cells, characterized by genetic and epigenetic modifications, exhibit deregulated cell functions [57]. The immune system can detect some of these abnormalities and eradicate cancer cells [58]. Tumor microenvironment (TME) modifications are also potential targets for the immune system [59]. Tumor-infiltrating lymphocytes (TILs) are lymphocytes attracted to and infiltrating the TME. Their presence is linked to better prognosis in cancer patients and they have been used in autologous adoptive cell therapy [60]. However, cancer cells, originating from mutated healthy cells, can be difficult for the immune system to detect [61].
The Chimeric Antigen Receptor (CAR) is an engineered TCR capable of binding to a target antigen. Introducing CARs into T lymphocytes creates CAR T cells that can detect specific antigens. CARs consist of the single chain variable Fragment (scFv) of a human IgG antibody. Three CAR generations exist: first-generation CARs combined an scFv with a CD3 signal for cytotoxic activity [62,63]. Second-generation CARs added a CD28 signal to promote proliferation and cytokine production [64]. Third-generation CARs included 4-1BB and OX40 regions to enhance survival and in vivo persistence [65]. Fourth-generation CARs, recently developed, contain specific cytokine signals, making CAR T cells resistant to TME immunosuppression and further improving in vivo lifespan [66,67]. These advancements in CAR design are as iterative and refined as the improvements in model t car prop tools vectors over time.
3.2. T Cell Therapy Bioprocessing: From Patient to Therapy
T cell adoptive therapy follows two main approaches based on cell source: allogeneic (universal donor cells) and autologous (patient-derived cells). Currently, all commercially available T cell therapies are autologous due to historical challenges with allogeneic approaches [68].
For most commercial and clinical CAR T cell therapies, cells are obtained from patient peripheral blood mononuclear cells (PBMCs) via apheresis or leukapheresis. This material includes B cells, macrophages, monocytes, NK cells, and T cells. The first step is isolating T cells from PBMCs, using magnetic bead selection or selective expansion [69,70]. Magnetic selection involves magnetic beads conjugated to antibodies targeting T cell populations (e.g., anti-CD3, anti-CD4/CD8) [14]. After selection, cells are rested in culture medium before transduction with a viral vector carrying the CAR cassette. This introduces the CAR gene, creating functional CAR T cells [71]. Transfection is an alternative to viral transduction, using pDNA with chemical transfection (e.g., polyethyleneimine (PEI)) or electroporation. However, these methods require large pDNA quantities and are less common in therapeutic applications [72].
Gene delivery for therapeutic CAR T cells can involve stable integration into T cell DNA, non-integrating transient transfer, viral vector-mediated transduction, or non-viral transduction [4]. To reach therapeutic cell doses, cells are cultured and expanded, typically activated with anti-CD3/CD28 antibody-conjugated beads [73]. Bioreactors can be used for expansion in closed systems with controlled conditions and quality monitoring [74].
CAR T cell therapy development faces challenges like poor cell expansion, short in vivo lifespan, and side effects [75]. Obtaining sufficient target T cells is crucial. Clinical material from cancer patients, often pre-treated with harsh therapies, is of lower quality and more variable than preclinical material from healthy donors. Patient sampling can also be detrimental [69]. Effective cell expansion technologies are therefore paramount for CAR T cell therapy success [14,76].
Safety is a significant concern with CAR T cell therapy, notably cytokine release syndrome (CRS) or “cytokine storm”. CRS is caused by T cell overactivation, resulting in massive pro-inflammatory factor release, leading to a positive feedback loop of immune cell activation. CRS symptoms can be severe and life-threatening [75,77]. Addressing these challenges is crucial to make CAR T cell therapy safer and more effective, just as continuous refinement is necessary for model t car prop tools vectors to meet evolving needs.
4. Applications of LV Vector Technologies in T Cell Engineering
4.1. Introduction to LV Applications
LV vectors are well-established in vitro research tools and are increasingly used in clinical applications. Their safety and efficacy are demonstrated in clinical trials [78], with some products gaining market authorization. Figure 3 shows the manufacturing process for commercial autologous therapies. However, issues like CRS, neurotoxicity, and cost need to be addressed for wider accessibility [75]. GMP-compliant tools and techniques are essential for LV-engineered T cell therapies, much like specialized tools are needed for intricate model t car prop restoration. This section explores LV vector technology applications in T cell engineering, including current CAR T cell therapies, integrated technologies, research tools, allogeneic approaches, and CAR T cell therapy alternatives.
Figure 3.
Alt Text: Viral Vector and Autologous CAR T Cell Therapy Bioprocessing: Diagram illustrating the parallel bioprocessing pathways for viral vectors and autologous CAR T cell therapy, akin to showcasing the complementary tools and processes involved in restoring a model t car, from vector production to patient infusion.
Viral vector and autologous CAR T cell therapy bioprocessing.
Viral vector bioprocessing begins with the expansion of the producer cell line, e.g., HEK293T cells. Cells are then transfected with plasmid DNA (pDNA) vectors. Post transfection, the viral particles produced are harvested from the cell supernatant. The clarification step is performed to remove large impurities such as aggregates and cell debris; this step may include a nuclease digestion step. The capture step aims at retaining the LV particles and further removes impurities. The polishing step removes impurities which are usually with similar physical properties to the LV vectors. The fill-finish step consists of the final formulation of the LV vector.
Autologous CAR T cell bioprocessing starts with the isolation of peripheral blood mononuclear cells (PBMCs) from the patient by apheresis or leukapheresis. To isolate the T lymphocytes from the PBMCs, an enrichment step can be introduced using antibody-conjugated magnetic beads. T cells are activated using anti-CD3/CD28 antibody-conjugated beads. Post-activation, cells are transduced using lentiviral vectors, and the CAR therapeutic gene is integrated in the target T cell genome to produce fully functional CAR T cells. The activated T cells are then expanded in culture to reach the required cell number for the therapy infusion course. The cell material is then harvested and conditioned for cryopreservation at −120 °C. The material is then shipped back to the clinic to be administered to the patient through intravenous infusion.
4.2. Commercially Available Gene-Modified T Cell Therapies
Of the three authorized CAR T cell therapies, brexucabtagene autoleucel (Tecartus, Kite Pharma Inc.) [9,10] and axicabtagene ciloleucel (Yescarta, Kite Pharma Inc.) [7,8] use GRV vectors, while tisagenlecleucel (Kymriah®, Novartis International AG) [5,6] uses an LV vector. Brexucabtagene autoleucel and axicabtagene ciloleucel use the same viral vector with the MSCV promoter [7,9]. Tisagenlecleucel uses a unique LV vector with the EF-1a promoter [5]. While other gene transfer techniques are being clinically assessed, retroviral vectors remain dominant for cell therapy applications, as reflected in the EMA and FDA approved therapies listed in Table 1.
Table 1.
EMA and FDA approved gene-modified T cell therapies (accessed on 4 January 2021).
| INN 1(Commercial Name) | Manufacturer | Application(s) | Therapy Type | Market Approval | Price per Dose (USD 4) | Reference |
|---|---|---|---|---|---|---|
| EMA 2 | FDA 3 | |||||
| brexucabtagene autoleucel (Tecartus®) | Kite Pharma Inc. (Gilead) | Mantle cell lymphoma | CAR T cellGRV vector | 2020 | 2020 | $ |
| tisagenlecleucel(Kymriah®) | Novartis AG | Acute B-celllymphoblastic leukaemia | CAR T cellLV vector | 2018 | 2017 | $ |
| axicabtagene ciloleucel(Yescarta®) | Kite Pharma Inc. (Gilead) | B cell lymphoma | CAR T cellGRV vector | 2018 | 2017 | $ |
1 International Non-proprietary Name—2 European Medicines Agency—3 Food and Drug Administration—4 United States Dollar.
Current autologous CAR T cell therapy protocols, as shown in Figure 3, are viable commercially but need improvement to meet global demand [14]. Due to cost and manufacturing capacity, LV-engineered T cell therapies are currently second-, third-, or fourth-line options for patients who have failed other treatments. Improving bioprocesses for both LV vectors and T cell engineering is crucial to enhance patient access. Recent advancements aim to address bottlenecks, improve functionality, and enhance safety of LV-engineered T cell therapies.
Logistics, including patient material sourcing, production, shipping, and storage, are major bottlenecks [79]. The 22-day bioprocess, often performed in the US, poses logistical challenges for other markets, like the EU. Novartis is expanding manufacturing in France and Switzerland to mitigate this [80].
EMA’s PRIority MEdicines scheme (PRIME) and FDA’s breakthrough therapy designation expedite market approval for drugs showing significant improvement over current standards [81]. All three CAR T cell therapies benefited from this accelerated evaluation due to their high efficacy for B cell malignancies and unmet clinical need [82,83].
Tisagenlecleucel received FDA and EMA approval for mantle cell lymphoma (MCL) [5,6]. T lymphocytes are modified with an anti-CD19 CAR using an LV vector and infused back into patients. Phase II (ELIANA, NCT02435849) and Phase III (BELINDA, NCT03570892) trials are ongoing [84,85,86]. Encouraging results from anti-CD19 CAR T cell therapy have spurred efforts to standardize protocols and anticipate clinical needs [18,87,88].
4.3. Limitations, Safety and Efficacy: Addressing Challenges
Despite success in blood cancers, CAR T cell therapy faces safety and efficacy challenges, especially in solid tumors.
Beyond separating LV genes into plasmids, safety modifications include “suicide genes” [89,90]. These allow selective killing of CAR T cells with a specific chemical agent. This has reached pre-clinical and clinical stages [91,92]. For example, anti-SLAMF7 therapy for multiple myeloma (MM) included a caspase-9 suicide gene to mitigate off-target activation, as SLAMF7 is on healthy leukocytes. Efficacy was shown in vitro and in vivo, and modified cells could be eliminated with AP1903 (rimiducid) [93].
Another risk is transgene transduction in non-target cells. Transduction of a single leukemia B cell led to a resistant transgenic clone in a tisagenlecleucel (Kymriah) patient [94], masking the CD19 epitope. While rare, broad tropism vectors (like VSV-G pseudotyped vectors [2]) can pose safety concerns. Engineering more specific chimeric envelope proteins, such as measles envelope-based proteins targeting CD3, could address this. In vivo data in humanized mice showed specificity and allowed in vivo gene delivery for CAR T cell transduction [95].
Relapse due to CD19 antigen loss in lymphoma B cells has also been reported [96,97]. Introducing two to three CAR constructs per cell is being investigated to prevent resistance, with encouraging results [98,99].
CAR expression levels impact clinical outcomes. Promoter selection and CAR transgene design, including transmembrane and hinge domains, can optimize CAR expression [100,101].
CAR T cell therapy has limited success in solid tumors [102]. Solid tumor properties and the TME hinder CAR T cell access and killing ability [103]. Obstacles include poor tumor penetration, harsh tumor conditions, and immunosuppressive proteins. These barriers are less significant in blood cancers, explaining the success of anti-CD19 CAR therapy.
Alternatives to LV and RV vectors include electroporation [104], which introduces pDNA using electric current, creating temporary pores. Non-integrating gene transfer improves safety, as transgene expression decreases over time. However, long-term efficacy needs to be demonstrated in human trials. Just as model t car prop tools vectors have limitations for modern use, LV vectors also face challenges that necessitate exploring alternatives and improvements.
4.4. Development of LV-Based Tools for CAR T Cell Therapy Research
Besides gene transfer, LV vectors are used to develop in vivo and in vitro models. Target cells expressing tumor-associated antigens (TAAs) are essential for CAR T cell therapy research. Immortalized cell lines, modified using LV vectors for stable TAA expression, are used to test CAR T cell performance in preclinical studies, replacing scarce patient material [105,106,107]. These modified cell lines are used for in vitro killing assays to demonstrate therapy potency and specificity [99,106], and can be engrafted to create in vivo tumor models for safety and efficacy studies [99]. Murine models are crucial for in vivo CAR T cell therapy testing, assessing efficacy and safety [108]. Mouse models include syngeneic immunocompetent, human xenograft, immunocompetent transgenic, and humanized transgenic models [109,110,111]. These models, infused with cancer cell lines, are essential for understanding tumor development and immune responses to therapy [108], providing preclinical evidence for safety and efficacy before human clinical trials [112]. LV vectors, like versatile prop tools, are instrumental in various stages of therapy development.
4.5. Development of Instruments Integrating LV to Current T Cell Bioprocesses
While autologous CAR T cell therapy is effective, challenges remain. Bioreactor manufacturers are designing GMP-compliant platforms for closed systems, specifically adapted for T cell bioprocessing, such as ambr 250 (Sartorius), Replicell™System (Aastrom Biosciences), Cocoon (Lonza), and Quantum Cell Expansion System (Terumo BCT) [113]. The CliniMACS Prodigy (Miltenyi) is a popular single-use, closed, GMP-compliant platform for T cell engineering, featuring a culture chamber with a centrifuge and viral vector application lines for integrated LV transduction [114,115,116]. Suitable for autologous approaches, it produces single doses per instrument, offering a scale-out solution for decentralized production closer to clinical sites [117]. On-site Phase I trials using CliniMACS Prodigy for dual anti-CD19/CD20 CAR T cell therapy for non-Hodgkin’s B-cell lymphomas demonstrated successful 14-day integrated manufacturing with LV transduction. All 8 patients received therapeutic doses meeting release criteria [118]. These technologies can improve product quality, production flexibility, reduce costs, and simplify logistics, much like specialized tools streamline the restoration of a model t car.
4.6. CAR T Cell Allogeneic Approach Using LV Vectors
Autologous CAR T cell therapy mitigates graft versus host disease (GvHD) risks [119], and avoids anti-HLA donor-specific antibodies (DSAs) [120,121]. However, it faces scale-up limitations due to patient material variability and low expansion potential [122,123], and bespoke production for each patient hinders large-scale manufacturing [69]. The allogeneic approach, using universal donor sources like genetically modified donor T cells, umbilical cord blood (UCB), placenta, immortalized cell lines, or iPSC-derived T cells, offers potential for large-scale production [124,125,126,127,128]. Donor T cells and immortalized cell lines have reached clinical stages, while stem cell-derived approaches are still in preclinical optimization [125,127].
The first in-human test of donor-derived allogeneic CAR T cell therapy (UCART19) in pediatric patients at Great Ormond Street Hospital in 2015 used lymphodepletion and TALEN gene editing to knockout CD52 and TCR genes, preventing GvHD. LV vectors introduced the anti-CD19 CAR gene. Molecular remission was achieved in both patients [125]. Cellectis licensed UCART19 to Servier, sponsoring Phase I trials completed in 2020, enrolling children and adults with B cell acute lymphoblastic leukemia. Results showed common adverse effects like CRS (91%) and neurotoxicity (38%), with two treatment-related deaths. Overall survival was 55%, progression-free survival 27% [129]. Cellectis, Allogene, and Servier are now investigating TCR and CD52 KO CAR T cells (ALLO-501) with anti-CD52 antibody (ALLO-647) for lymphoma treatment in a phase I trial (NCT03939026) [130]. Preliminary data showed a 63% objective response rate and 37% complete response [131].
Allogeneic T cell donor approaches, while promising, require additional steps to mitigate GvHD risk. The integration of LV vector technology in advanced allogeneic CAR T cell therapies underscores the importance of this gene transfer method in T cell engineering. Market approval of these therapies will further increase demand for clinical-grade LV vectors, highlighting the need for improved production processes, much like increased demand for model t car prop tools vectors necessitates efficient production methods.
4.7. Stable Production of LV Vectors to Address Increasing Vector Demand
To meet growing global demand, efforts focus on improving LV bioprocessing and developing technologies to increase production. Large pharmaceutical companies are investing in addressing bottlenecks. HEK293T cell line scale-up is a major restriction. Although transient transfection of adherent HEK293T cells achieves high titers, adherent nature limits conventional scale-up like stir tank bioreactors [132]. Stable production of LV vectors using dedicated packaging cell lines (PCLs) is suggested to reduce GMP-grade plasmid DNA costs in transient models [133]. Stable PCLs have viral genes stably transduced into their genome, and high-titer clones can be selected [33,134,135,136,137,138]. While promising, low titers compared to transient transfection remain a challenge [139]. Suspension variants of HEK 293T cells are also being explored [140]. Chen et al. (2020) combined suspension and stable aspects using a single stably-transfected construct, achieving LV titers comparable to transient systems, tested in stir tank bioreactors, with CAR T cell therapy as a potential application [141]. This technology, originally from TIGET and Cell Genesys and patented by GlaxoSmithKline (PCT/EP2016/078336), shows commercialization potential [141,142]. Like optimizing production for model t car prop tools vectors to meet collector demand, these advancements aim to enhance LV vector production capacity.
4.8. Dedicated LV Technology for CAR T Cell Therapy Applications
Developing dedicated LV particles for CAR T cell applications can improve therapy bioprocessing and function. T cell activation is crucial for high transduction efficiency with LV vectors [143]. Activation also leverages T cell clonal expansion for therapeutic cell numbers. This is typically done with anti-CD3/CD28 antibody-conjugated beads (Table 2) [69]. LentiSTIM and RetroSTIM technology (PCT/GB2016/050537) combine transduction and activation. LentiSTIM particles, produced in cell lines expressing membrane-bound anti-CD3/CD28 mitogens, carry these mitogens on their envelope [144]. Biotin groups on the LV envelope have also been used to improve purification [145]. This technology simplifies CAR T cell production by reducing steps and reagents and improving vector purity [146], similar to how specialized tools simplify car restoration.
Table 2.
GMP compliant T cell activation technologies.
| Product | Activation Method | Antibody Scaffold |
|---|---|---|
| Dynabeads (Gibco) | CD3/CD28Antibody-mediated | Magnetic beads |
| TransAct (Miltenyi Biotec) | CD3/CD28Antibody-mediated | Polymer beads |
| Cloudz (Bio-Techne) | CD3/CD28Antibody-mediated | Dissolvable beads |
4.9. Alternative Approaches to CAR T Cell Therapy Using LV Technology
TCR T cell therapy, using modified T cell receptors, is an alternative to CAR T cells for targeting malignancies [147]. TCR T cells use the same activation pathways as unmodified T cells, potentially reducing CRS and neurotoxicity [148]. Adaptimmune Therapeutics’ Specific Peptide Enhanced Affinity Receptor (SPEAR) technology uses LV vectors to manufacture TCR T cells with optimized TCRs for solid tumors [149]. Phase II trials like “redirected auto T cells for advanced myeloma” (NCT01352286) showed an 80% objective response rate at day 42 and 44% after 1 year [149,150]. SPEAR T cells are also in Phase II SPEAREAD 1 (NCT04044768) and 2 (NCT04408898) trials for synovial sarcoma, liposarcoma, and head and neck cancer [151]. T cells redirected for universal cytokine-mediated killing (TRUCKs), or fourth-generation CAR T cells, improve solid tumor efficacy through cytokine release, counteracting TME immunosuppression [152]. TRUCKs are in clinical trials, e.g., anti-EGFR-IL12-CART for metastatic colorectal cancer (NCT03542799) [153]. LV technology enables single-vector transduction for fully functional TRUCKs, enhancing their appeal [154].
Novel T cell subsets like γδ T cells, natural killer T (NKT) cells, and T regulatory (Treg) cells are promising alternatives, often engineered with LV technology [155,156]. γδ T cells show potent graft versus tumor (GVT) activity and low GVHD potential [157]. High γδ T cell levels are associated with better survival in HSCT patients [158]. Protocols and patents exist for LV-mediated γδ T cell culture and transduction, including serum-free media adaptations [159,160,161]. IN8BIO Inc.’s NC200 clinical trial is testing LV-modified γδ T cells for glioblastoma multiforme (GBM) [162].
Invariant natural killer T (iNKT) cells are another promising subset with strong anti-tumor activity and no GVHD risk, making them “off-the-shelf” candidates [163]. Anti-GD2 CAR iNKT cells (GRV vector-transduced) showed preclinical efficacy against neuroblastoma, leading to clinical trials [164,165]. Phase I study of CAR iNKTs (GINAKIT cells, NCT03294954) for neuroblastoma is ongoing [166], as is anti-CD19 CAR-iNKT therapy for B cell tumors (NCT04814004) [167].
Regulatory T cells (Tregs) control immune responses [168], with potential for treating autoimmune diseases and preventing GvHD [169,170]. Sangamo’s CAR Treg product TX200-KT02 (LV vector-transduced) is in Phase I/IIa trial (2019-001730-34) to prevent allograft rejection in ESRD patients [171]. These diverse applications of LV technology in T cell therapy demonstrate its versatility, much like model t car prop tools vectors can be adapted for various creative purposes.
5. Conclusions: LV Vectors as Cornerstones of T-Cell Therapy
LV vectors have been crucial in developing gene-modified T cell therapies, serving as safe and effective gene transfer tools for CAR T cell therapies. They are instrumental in transferring CAR genes and developing in vitro and in vivo testing models. Their importance is reflected in their integration into T cell therapy protocols and manufacturing instrument design. Clinical trial advancements, regulatory fast-track schemes, and industry involvement highlight the future significance of T cell therapies in oncology. While gene transfer alternatives exist, LV and GRV vectors are currently the most advanced methods for gene-modified T cell therapy manufacturing, much like model t car prop tools vectors remain central to historical representations.
Acknowledgments
The authors acknowledge funding and support from the UK Engineering and Physical Sciences Research Council (EPSRC) through the Future Targeted Healthcare Manufacturing Hub at University College London (Grant Reference: EP/P006485/1), and industrial consortium support.
Author Contributions
Conceptualization, R.P.L.; investigation, R.P.L.; writing—original draft preparation, R.P.L.; writing—review and editing, R.P.L., S.V. and Q.A.R.; supervision, S.V. and Q.A.R.; project administration, S.V. and Q.A.R.; funding acquisition, Q.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was secured from the UK Engineering and Physical Sciences Research Council (EPSRC) (Grant Reference: EP/P006485/1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This review article cited all material in text and bibliography, without original data.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral regarding jurisdictional claims.
References
Associated Data
Data Availability Statement
This review article cited all material in text and bibliography, without original data.

