Introduction
Pain is a significant yet often underestimated issue for individuals living with dementia. As cognitive decline progresses, especially in moderate to severe stages, the ability to self-report pain diminishes, leaving healthcare providers and caregivers to rely on observational cues. This communication barrier can lead to under-detection and subsequent undertreatment of pain, negatively impacting the quality of life for dementia patients and potentially exacerbating behavioral disturbances. Often, these behavioral changes, stemming from unaddressed pain, are mismanaged with psychotropic medications, creating further complications.
Recognizing non-verbal signals as crucial indicators of pain, organizations like the American Geriatric Society (AGS) have advocated for their inclusion in behavioral pain assessment tools. However, traditional observational tools are often hampered by their reliance on subjective interpretation and the varying expertise of assessors, particularly in settings with high staff turnover like aged care facilities. The need for more objective and reliable pain assessment methods in dementia care is clear.
Facial expressions are a critical component of non-verbal pain communication, and are frequently incorporated into observational pain scales. Yet, the descriptors used in these scales are often ambiguous and open to interpretation. The Facial Action Coding System (FACS) offers a more standardized and objective approach to analyzing facial expressions, breaking them down into specific Action Units (AUs). While FACS is considered the gold standard for facial expression analysis, its manual application is time-consuming and requires specialized training, making it impractical for routine clinical use. However, advancements in automated facial recognition technology offer a promising solution to bridge this gap.
Research indicates that individuals with Alzheimer’s disease, the most prevalent form of dementia, exhibit increased facial activity, including pain-related Action Units, possibly due to a reduced ability to suppress negative expressions. This underscores the potential of leveraging facial recognition technology for pain assessment in this population.
This article explores the critical need for effective Pain Assessment Tools In Dementia Care and introduces the electronic Pain Assessment Tool (ePAT), an innovative solution that integrates automated facial recognition technology. We will delve into the development, features, and psychometric evaluation of ePAT, comparing it to the widely used Abbey Pain Scale (APS), to demonstrate its potential to revolutionize pain management for individuals with dementia.
Methods: Evaluating Pain Assessment Tools
To rigorously assess the efficacy of pain assessment tools, particularly in the context of dementia care, robust methodologies are essential. This section details the tools used in a comparative study – the Abbey Pain Scale (APS) and the electronic Pain Assessment Tool (ePAT) – and the framework for evaluating their performance.
Abbey Pain Scale (APS): A Standard Observational Tool
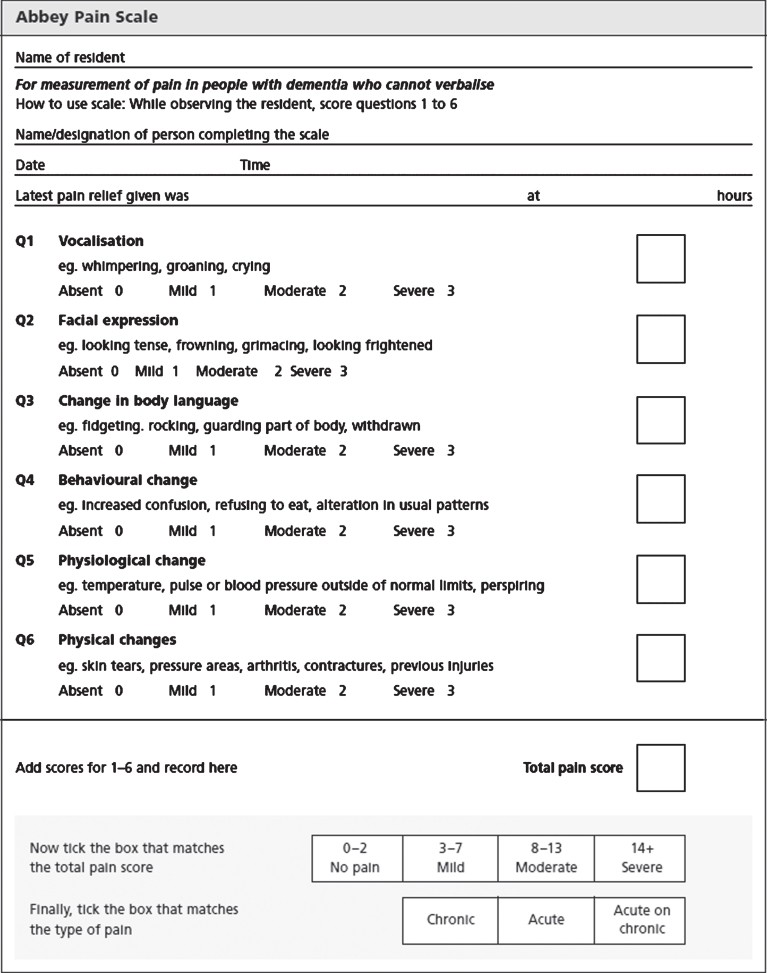
The Abbey Pain Scale (APS) served as the comparator in this evaluation due to its established use in clinical practice and endorsement by bodies like the Australian Pain Society. Its psychometric properties in older adults with dementia are well-documented in numerous systematic reviews. The APS encompasses six domains: vocalization, facial expressions, body language changes, behavioral changes, physiological changes, and physical changes. Each domain is scored on a 0-3 ordinal scale, representing pain intensity from absent (0) to severe (3). The total APS score, ranging from 0 to 18, categorizes pain levels from no pain to severe pain. The APS provides a structured observational framework, but its reliance on subjective scoring highlights the need for more objective measures.
Figure 1: The Abbey Pain Scale
The Abbey Pain Scale is a widely used tool for assessing pain in people with dementia. It relies on observing and scoring six domains of pain indicators.
Electronic Pain Assessment Tool (ePAT): Integrating Technology for Objective Assessment
The electronic Pain Assessment Tool (ePAT) is designed as a point-of-care solution to aid clinicians and caregivers in assessing pain in individuals with moderate to severe dementia. Developed through a collaboration between Curtin University and nViso SA, ePAT leverages automated facial recognition technology to objectively detect facial micro-expressions indicative of pain. This is combined with behavioral and physical features across five additional domains, aligning with the AGS framework for pain assessment.
ePAT analyzes a brief 10-second video of a patient’s face, using facial mapping to identify and quantify pain-related facial micro-expressions in real-time. These micro-expressions are based on the Facial Action Coding System (FACS), providing a standardized and anatomically detailed analysis.
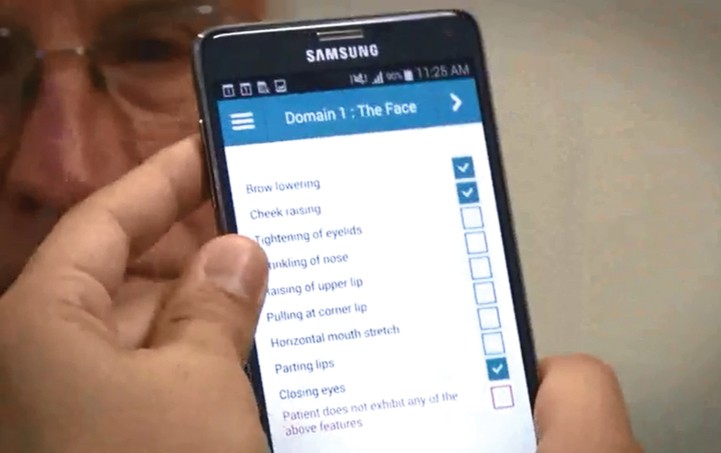
Image 1: Face detection and tracking in the ePAT App
ePAT utilizes face detection and tracking technology to ensure accurate facial expression analysis during pain assessment.
Image 2: Facial features extraction of the ePAT App
The ePAT app extracts key facial features to analyze subtle changes related to pain expressions.
Image 3: Detection of facial Action Units (AUs) codes in the ePAT App
ePAT automatically detects and codes facial Action Units (AUs) associated with pain, based on the Facial Action Coding System (FACS).
In total, ePAT encompasses 42 descriptor items across six domains: Face (9 items), Voice (9 items), Movement (7 items), Behavior (7 items), Activity (4 items), and Body (6 items). These domains cover a comprehensive range of pain indicators. Unlike the APS’s ordinal scoring, ePAT employs a binary (yes/no) format for each item, enhancing objectivity.
Image 4: Domain 5 of the ePAT; The Activity
Domain 5 of ePAT, “The Activity,” assesses changes in routine activities that may indicate pain.
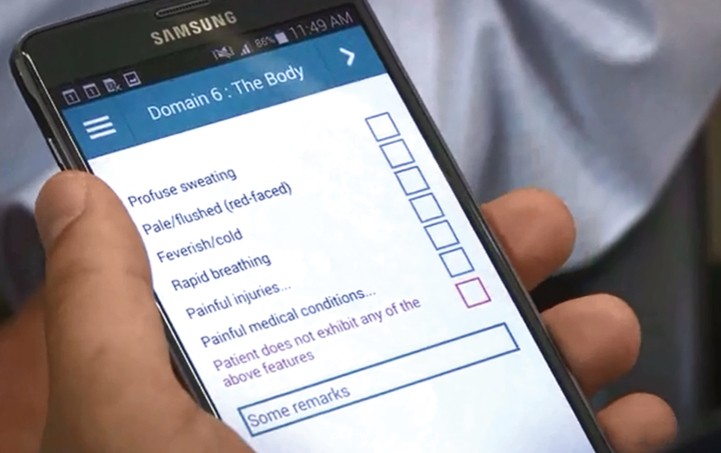
Image 5: Domain 6 of the ePAT; The Body
Domain 6 of ePAT, “The Body,” includes physical indicators like sweating, rapid breathing, and known painful conditions.
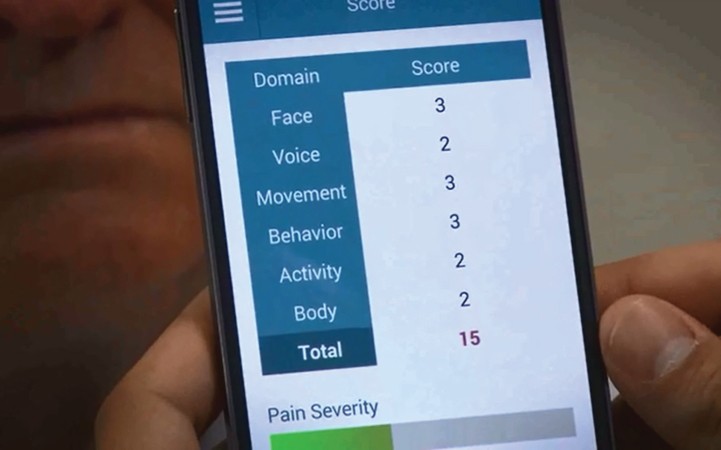
Image 6: Total score screen of the ePAT App
The ePAT app provides a total pain score, aiding in quick interpretation of pain intensity.
The ePAT app, designed for use on mobile smart devices, automates facial expression analysis while allowing manual input for the other five domains via a touch-screen interface. The total ePAT score ranges from 0 to 42. ePAT’s conceptual foundation is rooted in the definition of pain as a sensory and emotional experience linked to tissue damage and incorporates recommendations for FACS-based pain expression assessment in dementia care.
ePAT’s design principles prioritize:
- Objectivity: Through automated FACS integration, deep learning algorithms to minimize subjective error, and binary scoring for non-facial cues.
- Comprehensiveness: Inclusion of AGS items relevant to dementia patients to capture subtle behavioral pain indicators.
- Portability and Interoperability: Utilizing smart device technology for point-of-care accessibility across various platforms.
The following table provides a detailed comparison between APS and ePAT:
Table 1: Comparison between APS and ePAT
| Feature | Abbey Pain Scale (APS) | Electronic Pain Assessment Tool (ePAT) |
|---|---|---|
| Number of Domains | 6 | 6 |
| Tool Item Domains & Descriptors | Vocalization (1 item: whimpering, groaning, crying) Facial Expressions (1 item: tense, frowning, grimacing, frightened) Body Language (1 item: fidgeting, rocking, guarding, withdrawn) Behavioral Change (1 item: increased confusion, refusing to eat, altered patterns) Physiological Change (1 item: temperature, pulse, blood pressure, perspiring, flushing, pallor) Physical Change (1 item: skin tears, pressure areas, arthritis, contractures, injuries) |
Face (9 items: FACS Action Units) Voice (9 items: noisy pain sounds, requests for help, groaning, crying) Movement (7 items: altered movement, restlessness, guarding, pacing) Behavior (7 items: unsocial, offensive, aggressive, confused, distressed) Activity (4 items: resisting care, prolonged resting, altered sleep) Body (6 items: sweating, pale/flushed, feverish/cold, rapid breathing, injuries, conditions) |
| Facial Expression Assessment | Abstract description (tense, frowning, grimacing) | Specific annotation using FACS Action Units (AU4, AU6, AU7, AU9, AU10, AU12, AU20, AU25, AU43) |
| Scoring Format | Ordinal (4-point scale per domain: 0-3) | Binary (2-point scale per item: 0 or 1) |
| Scoring Procedure | Manual pen-and-paper recording | Automated facial recognition (Face domain) & touch-screen electronic completion (other domains) |
| Total Pain Score Range | 0-18 | 0-42 |



Results: ePAT Demonstrates Strong Psychometric Properties
The study evaluating ePAT involved 40 residents with moderate to severe dementia from three aged care facilities. The demographic characteristics of the participants, including age, gender, dementia type, and pre-existing pain conditions, were comprehensively documented. A total of 353 paired pain assessments using both APS and ePAT were conducted across various conditions (rest and movement) and by different assessors, including nurses, care workers, health science students, and the primary investigator.
Concurrent Validity: ePAT Aligns Closely with APS
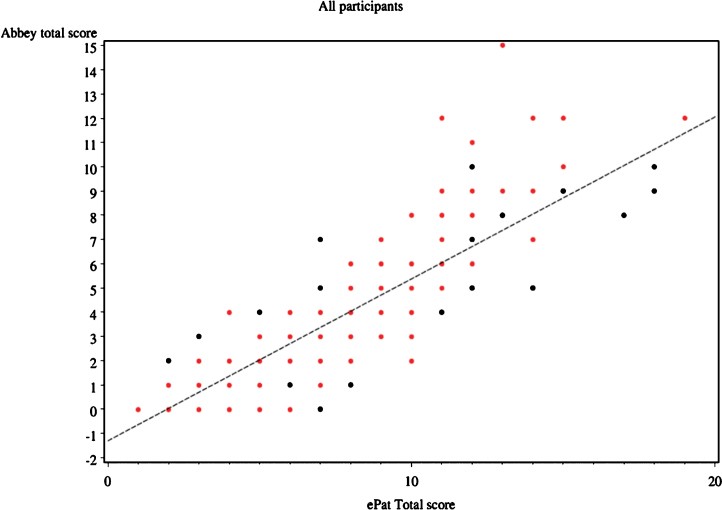
The concurrent validity of ePAT was assessed by comparing its pain scores with those of APS. The Pearson’s correlation coefficient for overall agreement between the two tools was remarkably high at 0.882 (95% CI: 0.857–0.903). This strong positive correlation indicates a very close relationship between the pain scores generated by ePAT and APS, suggesting that ePAT effectively measures pain in a manner consistent with the established APS. This significant correlation was maintained both during periods of rest (r=0.880) and with movement (r=0.894).
Figure 2: Scatter plot of APS vs ePAT scores
This scatter plot demonstrates the strong correlation between ePAT and APS scores, indicating good concurrent validity. Black dots represent assessments at rest, and red dots represent assessments during movement.
Discriminant Validity: ePAT Differentiates Pain Conditions
Discriminant validity was examined by assessing ePAT’s ability to differentiate pain scores between rest and movement conditions. Similar to APS, ePAT scores increased when residents experienced movement-related pain. Statistical analysis revealed that the timing of assessment (rest vs. movement) did not significantly influence the agreement between ePAT and APS scores (p=0.795). This indicates that ePAT, like APS, is sensitive to changes in pain levels associated with different conditions, demonstrating good discriminant validity.
Inter-rater Reliability: Consistent Pain Categorization
Inter-rater reliability, crucial for ensuring consistent application of pain assessment tools, was evaluated by categorizing pain scores from both APS and ePAT into four groups: no pain, mild, moderate, and severe. Weighted kappa scores were calculated to measure the agreement between these categorized scores. The overall weighted kappa score was 0.74 (95% CI: 0.69–0.80), indicating good inter-rater reliability. Reliability remained consistently good both at rest (weighted kappa=0.71) and with movement (weighted kappa=0.78).
Table 5: Agreement in Pain Categorization between APS and ePAT
| APS Category | ePAT Category | Total |
|---|---|---|
| No Pain | Mild | |
| No Pain | 183 (95.3%) | 9 (4.7%) |
| Mild | 32 (23.4%) | 97 (70.8%) |
| Moderate | 0 | 5 (21.7%) |
| Severe | 0 | 0 |
Table 6: Inter-rater Reliability (Weighted Kappa)
| Activity | Weighted Kappa | 95% CI |
|---|---|---|
| All (Rest + Movement) | 0.74 | 0.69–0.80 |
| At Rest | 0.71 | 0.63–0.80 |
| With Movement | 0.78 | 0.70–0.86 |
Internal Consistency: ePAT and APS Measure a Similar Construct
Internal consistency, assessing whether ePAT and APS measure a similar underlying construct, was evaluated using Cronbach’s alpha. The Cronbach’s alpha value was excellent at 0.925, with a Pearson’s correlation coefficient of r=0.882. This high internal consistency further supports the validity of ePAT as a pain assessment tool comparable to APS.
Discussion: ePAT – A Step Forward in Dementia Pain Management
The study findings robustly demonstrate that ePAT is a valid and reliable tool for pain assessment in individuals with moderate to severe dementia who are unable to self-report. ePAT offers notable advantages over existing behavioral pain assessment tools, primarily through its integration of automated facial recognition technology. This feature significantly reduces subjectivity in assessing facial expressions, a key domain in pain evaluation. The binary scoring system further contributes to objectivity and ease of use, providing a reproducible assessment of pain. Furthermore, ePAT’s app-based format facilitates efficient pain scoring and ongoing patient monitoring.
The strong concurrent validity, discriminant validity, inter-rater reliability, and internal consistency demonstrated by ePAT in this study are highly encouraging. The correlation coefficient of 0.88 between ePAT and APS surpasses the generally accepted threshold for new pain assessment tools, indicating a high level of agreement. Compared to other head-to-head evaluations of observational pain scales, ePAT’s performance is notably strong.
ePAT’s ability to differentiate pain scores between rest and movement conditions, similar to APS, highlights its sensitivity to changes in pain states. The good inter-rater reliability, with weighted kappa scores above 0.7, indicates that ePAT can be consistently applied by different assessors. The excellent internal consistency further validates that ePAT and APS are measuring a similar underlying construct of pain.
Strengths and Limitations
This study is a pioneering effort in evaluating a pain assessment tool incorporating automated facial recognition for dementia patients. The real-world setting of pain assessments during routine care adds to the ecological validity of the findings. However, limitations include a relatively small sample size, a homogenous sample predominantly of Caucasian females, and a non-random participant selection. Subjectivity in scoring non-facial domains of ePAT and all domains of APS, and the lack of a definitive gold standard for observational pain scales, are inherent challenges in this type of research. The observational nature of the study also means that the influence of potential confounding factors, such as underlying mental health conditions mimicking pain behaviors, cannot be entirely excluded.
Future Directions and Implications
Despite these limitations, ePAT shows significant promise for improving pain assessment and management in dementia care. Future research should explore ePAT’s responsiveness to pain interventions, both pharmacological and non-pharmacological. Further studies with larger and more diverse samples are needed to generalize these findings and to refine ePAT’s pain intensity categories. Additionally, investigating the usability and acceptance of ePAT by caregivers and healthcare professionals in routine clinical practice is crucial for its successful implementation.
Conclusion
The electronic Pain Assessment Tool (ePAT) represents a significant advancement in pain assessment for individuals with moderate to severe dementia. By integrating automated facial recognition with a comprehensive assessment of non-verbal pain cues, ePAT offers a more objective, reliable, and efficient approach compared to traditional observational tools. Its strong psychometric properties, as demonstrated in this study, support its potential to enhance pain management, improve patient comfort, and reduce the inappropriate use of psychotropic medications in dementia care settings. ePAT holds promise as a valuable tool for healthcare professionals and caregivers seeking to effectively address the often-overlooked issue of pain in people living with dementia.
Acknowledgments
The authors gratefully acknowledge the funding and support from Alzheimer’s Australia and the Alzheimer’s Australia Dementia Research Foundation. They also extend their sincere thanks to the aged care staff, residents, and families who participated in this research.
