The escalating opioid epidemic casts a long shadow, particularly on our youngest and most vulnerable: newborns. Infants born to mothers struggling with opioid use are increasingly affected by Neonatal Opioid Withdrawal Syndrome (NOWS), a condition requiring specialized care and attention. Like a finely tuned engine requiring precise diagnostic tools, these infants need effective assessment methods to ensure they receive the best possible start in life. This article delves into the critical need for improved NOWS management and highlights the promise of the eating, sleeping, consoling care tool, a groundbreaking approach poised to transform neonatal care.
The Growing Challenge of Neonatal Opioid Withdrawal Syndrome
The surge in opioid use among women of childbearing age has created a significant public health crisis. In the United States, opioid prescriptions were alarmingly common, with millions dispensed annually. This widespread availability, coupled with the rise in illicit opioid abuse, has led to a dramatic increase in opioid use disorders among new mothers. The numbers are stark: rates of opioid use disorders in new mothers have quadrupled in recent years, directly contributing to a fivefold increase in NOWS cases. This crisis disproportionately impacts rural and underserved communities, mirroring areas where access to specialized healthcare and resources may already be strained, much like access to specialized car repair in remote areas.
The consequences for infants are profound. NOWS can manifest in varying degrees of severity, impacting a newborn’s fundamental abilities – their ability to eat, sleep, and be consoled. Some infants experience mild symptoms, while others require intensive pharmacological intervention to mitigate the negative effects on their development, similar to how some car problems are minor while others require major repairs to prevent long-term damage. The challenge lies in accurately assessing the severity of withdrawal and guiding appropriate treatment, a process that, until recently, has relied on subjective and potentially flawed tools.
Limitations of Traditional Assessment Methods: The FNAST
For decades, the Finnegan Neonatal Abstinence Scoring Tool (FNAST) has been the predominant method for assessing NOWS in the United States. Used in the vast majority of institutions, the FNAST aims to quantify withdrawal severity and guide pharmacotherapy. However, this tool, developed in the 1970s, has significant limitations. Like using outdated diagnostic equipment on a modern vehicle, the FNAST may not be as effective or accurate in today’s context.
Concerns surrounding the FNAST include:
- Subjectivity: The FNAST relies on observer-rated scores, introducing inherent subjectivity in assessments. Just as different mechanics might interpret car symptoms slightly differently, clinical interpretations of FNAST criteria can vary.
- Disruption: Formal FNAST assessments can be disruptive to infants, requiring them to be disturbed, which can be counterproductive to their recovery, similar to unnecessary invasive diagnostic procedures on a car that could cause further issues.
- Overestimation of Need for Medication: Critically, the FNAST may overestimate the need for pharmacological therapy. By incorporating all signs of withdrawal, including clinically insignificant ones, it can lead to unnecessary medication use, prolonged hospital stays, and increased healthcare costs, akin to recommending major engine work when a minor adjustment would suffice.
This overestimation has significant implications, contributing to longer hospital stays and increased financial burdens on healthcare systems, especially those serving vulnerable populations already facing economic hardship. The need for a more refined, accurate, and less invasive assessment tool is clear, much like the automotive industry constantly seeks better diagnostic tools for efficient and effective car care.
Introducing the Eating Sleeping Consoling (ESC) Care Tool: A Functional Approach
In response to the limitations of the FNAST, the Eating Sleeping Consoling Care Tool, also known as the ESC Care Tool, has emerged as a promising alternative. This innovative approach, rooted in quality improvement initiatives, shifts the focus from solely scoring withdrawal signs to evaluating an infant’s functional abilities – their capacity to eat, sleep, and be consoled. This is akin to assessing a car’s performance based on how it actually drives and functions, rather than just relying on error codes.
The ESC Care Tool operates on a simple yet profound principle: if an infant is effectively eating (feeding well within a reasonable timeframe), sleeping (undisturbed for at least an hour), and consolable (calmed within 10 minutes), pharmacological intervention is not immediately necessary. This function-based assessment emphasizes non-pharmacological care as the first-line treatment, empowering families and caregivers to play a central role in the infant’s recovery. Just as preventative maintenance and careful driving can minimize the need for major car repairs, non-pharmacological interventions like skin-to-skin contact, breastfeeding support, and a calm environment are prioritized in the ESC approach.
When challenges arise in eating, sleeping, or consoling, the care team first focuses on optimizing these non-pharmacological strategies. Pharmacological therapy is only initiated or escalated if these interventions prove insufficient, ensuring medication is used judiciously and only when truly needed. This measured approach contrasts sharply with the FNAST, which can trigger medication based on scores alone, potentially overlooking the effectiveness of non-pharmacological care.
Evidence of Effectiveness: The ESC Approach in Practice
The ESC approach, emphasizing parental involvement and simplified assessment, originated at Yale-New Haven Children’s Hospital and has demonstrated remarkable results. Over a five-year quality improvement period, the proportion of methadone-exposed infants receiving pharmacological treatment for NOWS plummeted from 98% to a mere 14%. This dramatic reduction was achieved without any reported seizures or readmissions related to withdrawal, showcasing the safety and efficacy of the ESC approach. This is comparable to finding a more efficient and reliable car repair method that significantly reduces the need for costly and invasive procedures.
While the initial pharmacologic treatment rate at Yale was exceptionally high compared to national averages, a direct comparison further solidified the ESC Care Tool’s potential. In a retrospective study comparing ESC-based management to FNAST-based decisions, morphine initiation was indicated for only 12% of infants managed with ESC, compared to 62% based on FNAST scores. Again, no adverse events or readmissions were reported, reinforcing the ESC approach’s safety and its ability to significantly reduce unnecessary pharmacological interventions.
Other institutions have replicated these promising findings. One institution implementing a pilot version of the ESC Care Tool observed a substantial decrease in pharmacological treatment from 87% to 40% and a reduction in hospital length of stay from 17 to 11 days, with no adverse events. These consistent results across different settings highlight the generalizability and effectiveness of the ESC Care Tool in diverse healthcare environments.
Standardizing and Expanding the ESC Care Tool
Recognizing the potential of the ESC approach, efforts have been undertaken to standardize its implementation through the development of a formal ESC Care Tool and comprehensive training materials. Initial evaluations of the ESC Care Tool’s assessment component have demonstrated high inter- and intra-rater reliability, ensuring consistent and reliable assessments across different healthcare providers, much like standardized diagnostic procedures in auto repair shops ensure consistent service quality.
Collaborative efforts between leading institutions have produced standardized training materials, including instructional manuals, the ESC Care Tool with clear definitions, newborn care diaries, training videos, and case scenarios. These resources facilitate effective training and implementation, even in smaller community hospitals, ensuring that the benefits of the ESC approach can reach infants in all settings, particularly in rural and underserved areas where the opioid crisis is most acutely felt.
The Need for Rigorous Study and Future Directions
While the outcomes following ESC Care Tool implementation are encouraging, rigorous scientific study is crucial to definitively establish its safety, efficacy, and generalizability. Current reports primarily come from hospitals with high levels of maternal compliance with medication-assisted treatment and strong family support systems. The long-term effects of ESC care on infant and family well-being after discharge also remain important areas for investigation.
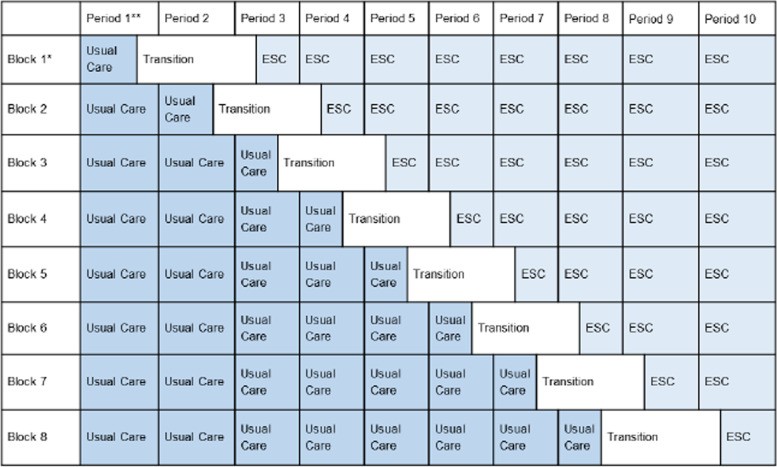
To address these gaps, a stepped-wedge cluster randomized controlled trial is proposed to compare the ESC care approach to usual care with the FNAST. This study design, involving multiple US sites, will provide robust evidence on the short- and long-term outcomes of infants managed with the ESC Care Tool, moving towards an evidence-based standard of care for NOWS management, similar to how automotive engineering research leads to improved vehicle maintenance and diagnostic procedures.
Primary Hypothesis: The ESC care approach will reduce the length of time until infants are medically ready for discharge by an average of 4 days compared to usual FNAST-based care.
Secondary Hypothesis: The ESC care approach will improve infant neurobehavioral functioning and family well-being compared to usual FNAST-based care.
These hypotheses are grounded in the potential of the ESC approach to safely reduce hospital stays, minimize unnecessary opioid exposure, and enhance maternal-infant bonding. By focusing on functional abilities and empowering families, the ESC Care Tool has the potential to not only improve immediate neonatal outcomes but also foster long-term well-being for infants and families affected by NOWS.
Conclusion: A Transformative Tool for Neonatal Care
The eating sleeping consoling care tool represents a significant advancement in the management of Neonatal Opioid Withdrawal Syndrome. By shifting the focus to functional assessment and prioritizing non-pharmacological care, this approach offers a more humane, effective, and cost-efficient alternative to traditional methods like the FNAST. Just as innovative diagnostic tools have revolutionized car repair, the ESC Care Tool promises to transform neonatal care, ensuring that infants affected by the opioid crisis receive the individualized, family-centered care they need to thrive. Further research and widespread implementation of this care tool are essential steps towards mitigating the devastating impact of the opioid epidemic on newborns and building healthier futures for vulnerable families.
Table 1: Stepped-wedge cluster randomized controlled trial with transition period, illustrating the randomized rollout of the ESC care approach across different blocks of study sites over time.
Visual representation of the stepped-wedge study design, highlighting the transition periods and staggered implementation of the ESC care approach across participating sites.