Introduction
Adherence to antiretroviral therapy (ART) is paramount for the successful management of HIV infection. Consistent ART adherence is directly linked to improved virologic control, stronger immune responses, and better clinical outcomes, including reduced disease progression and mortality. Accurately measuring ART adherence, however, presents significant challenges. Despite the critical need, a universally accepted standard for adherence measurement remains elusive. Furthermore, the effectiveness of different measurement methods across diverse patient populations and clinical settings requires ongoing evaluation. While various approaches exist, ranging from direct measures like observed therapy to indirect methods such as pill counts and self-reports, many are impractical for routine clinical use due to their complexity, cost, or burden on patients and providers.
Self-report methods offer a practical alternative, being quick, inexpensive, and providing valuable insights into the patient’s perspective on their medication-taking behavior. Although self-reports may sometimes overestimate adherence, numerous studies have demonstrated a robust correlation between self-reported adherence and positive health outcomes in individuals living with HIV. These outcomes include viral suppression, improved immune function, and enhanced quality of life.
One commonly employed self-report method is the three-day self-report, often utilized within instruments like the Adult AIDS Clinical Trials Group (AACTG) questionnaire. This method involves detailing medication use over the preceding three days. While effective in research settings, it can be time-consuming, reliant on patient recall, and may require staff assistance. Simpler, more efficient tools are needed to streamline adherence assessment in routine clinical practice.
This article focuses on the Mannheimer Aids Care Adherence Screening Tool, also known as the CASE Adherence Index, a concise and easily administered self-report instrument designed to address this need. Developed and evaluated by the Center for Adherence Support Evaluation (CASE), this index offers a practical approach for assessing ART adherence in diverse clinical settings. This article will delve into the development, validation, and clinical utility of the Mannheimer AIDS Care Adherence Screening Tool, highlighting its potential to enhance HIV care.
What is the Mannheimer AIDS Care Adherence Screening Tool?
The Mannheimer AIDS Care Adherence Screening Tool, or CASE Adherence Index, is a composite measure specifically designed for efficient and practical assessment of self-reported ART adherence. It was developed as part of a large cross-site evaluation study across 12 adherence support programs in the United States. This study aimed to evaluate interventions designed to improve ART adherence, particularly among underserved populations. Recognizing the need for a simple yet effective adherence measure, the CASE researchers developed this index.
Background and Development
The development of the Mannheimer AIDS Care Adherence Screening Tool was driven by the need for a practical adherence measure suitable for both research and clinical settings. The researchers at CASE sought to create an index that was:
- Brief: Easy and quick to administer, minimizing burden on patients and staff.
- Simple to Score: Straightforward scoring system for immediate results.
- Reliable: Capable of providing consistent and dependable adherence information.
- Valid: Accurately reflecting actual adherence behavior and correlating with relevant clinical outcomes.
The tool was developed using data from a large, multi-site study funded by the Health Resources and Services Administration (HRSA). This study involved diverse populations and various adherence support interventions, ensuring the tool’s applicability across different settings and patient demographics. The CASE Adherence Index emerged from analyses of various self-reported adherence questions, aiming to identify a concise set of questions that effectively captured key dimensions of adherence.
Components of the CASE Adherence Index
The Mannheimer AIDS Care Adherence Screening Tool comprises three straightforward self-report questions, each addressing a distinct aspect of ART adherence. These questions are:
A1. Difficulty Taking HIV Medications on Time: This question assesses the frequency with which patients experience difficulty taking their medications within the prescribed time window (no more than two hours before or after the scheduled time). Response options are:
- Never
- Rarely
- Most of the time
- All of the time
A2. Average Days per Week Missed Doses: This question queries the average number of days per week a patient misses at least one dose of their HIV medications. Response options are:
- Every day
- 4–6 days/week
- 2–3 days/week
- Once a week
- Less than once a week
- Never
A3. Last Time Missed a Dose: This question inquires about the recency of the last missed dose of HIV medication. Response options are:
- Within the past week
- 1–2 weeks ago
- 3–4 weeks ago
- Between 1 and 3 months ago
- More than 3 months ago
- Never
These three questions were selected based on correlation and principal component analyses, indicating that they measure related but distinct facets of adherence behavior. The index score is calculated by summing the points assigned to each response, with higher scores indicating better adherence. The scoring system is detailed in the Appendix.
Caption: The Mannheimer AIDS Care Adherence Screening Tool questionnaire, highlighting the three key questions (A1, A2, A3) used to assess ART adherence.
Validation and Methodology: Comparing the Mannheimer Tool to the Three-Day Self-Report
To establish the validity of the Mannheimer AIDS Care Adherence Screening Tool, researchers compared it to the AACTG three-day self-report, a more detailed and established adherence measure. The study also examined the index’s ability to predict virologic and immunologic outcomes in people living with HIV.
Study Design and Participants
The validation study was conducted as a prospective, longitudinal, cross-site evaluation. It involved 12 different adherence programs across the United States, representing a diverse range of geographic locations and clinical settings. Participants were adults living with HIV who were enrolled in these adherence support programs. They were antiretroviral-experienced, meaning they had prior experience with ART.
The study aimed to enroll individuals from underserved populations who often face significant barriers to ART adherence. The interventions implemented at each site varied, reflecting best practices and common approaches to adherence support, including counseling, case management, and directly observed therapy. Despite the diversity in interventions and settings, a standardized evaluation protocol was used across all sites, ensuring consistent data collection and analysis.
Data Collection and Measures
Data were collected through participant interviews and medical chart reviews at baseline and at 3, 6, 9, and 12-month intervals. Standardized questionnaires were used to gather information on sociodemographics and self-reported ART adherence, including both the Mannheimer AIDS Care Adherence Screening Tool and the three-day self-report. Medical chart abstractions provided data on HIV RNA levels and CD4 counts, key indicators of HIV disease progression and treatment effectiveness.
The three-day self-report, adapted from the AACTG instrument, involved a detailed day-by-day recall of doses taken and missed for each ART medication over the preceding three days. This yielded an overall adherence rate, often categorized as ≥95% adherence or <95% adherence, based on established thresholds for virologic success.
The Mannheimer AIDS Care Adherence Index was scored based on the summed responses to the three questions (A1, A2, A3). A cut-off score of 10 was identified as optimal for differentiating between good and poor adherence based on its sensitivity and specificity compared to the three-day self-report.
Statistical Analysis
Statistical analyses were conducted to assess the Mannheimer AIDS Care Adherence Screening Tool’s reliability and validity. This included:
- Correlation analysis: Examining the relationships between the CASE Adherence Index, the three-day self-report, and individual components of the CASE index.
- Sensitivity and Specificity analysis: Evaluating the ability of the CASE Adherence Index to correctly identify adherence levels as defined by the three-day self-report.
- Logistic Regression: Assessing the association between adherence measures and virologic outcomes (HIV RNA suppression).
- Receiver Operating Characteristic (ROC) curves: Comparing the predictive accuracy of the CASE Adherence Index and the three-day self-report for virologic suppression.
- T-tests: Analyzing differences in CD4 count changes between groups defined by adherence levels as measured by both tools.
These analyses aimed to determine how well the simpler Mannheimer tool aligned with the more complex three-day self-report and, more importantly, how effectively both measures predicted clinical outcomes.
Key Findings: The Efficacy of the Mannheimer Adherence Screening Tool
The study yielded significant findings supporting the validity and utility of the Mannheimer AIDS Care Adherence Screening Tool.
Correlation with Three-Day Self-Report
The Mannheimer AIDS Care Adherence Screening Tool demonstrated a strong correlation with the three-day self-report. Statistical analysis revealed:
- Significant Association: Participants classified as adherent by the Mannheimer tool (score >10) were significantly more likely to be classified as adherent (>95%) by the three-day self-report. The odds of having a >95% three-day self-report adherence score were substantially higher (at least 60 times) for those with a Mannheimer score >10.
- High Sensitivity and Specificity: Using a cut-off score of 10, the Mannheimer tool achieved a balance of sensitivity and specificity in identifying adherence as measured by the three-day self-report. This indicates that the tool effectively identifies both truly adherent and non-adherent individuals relative to the more detailed measure.
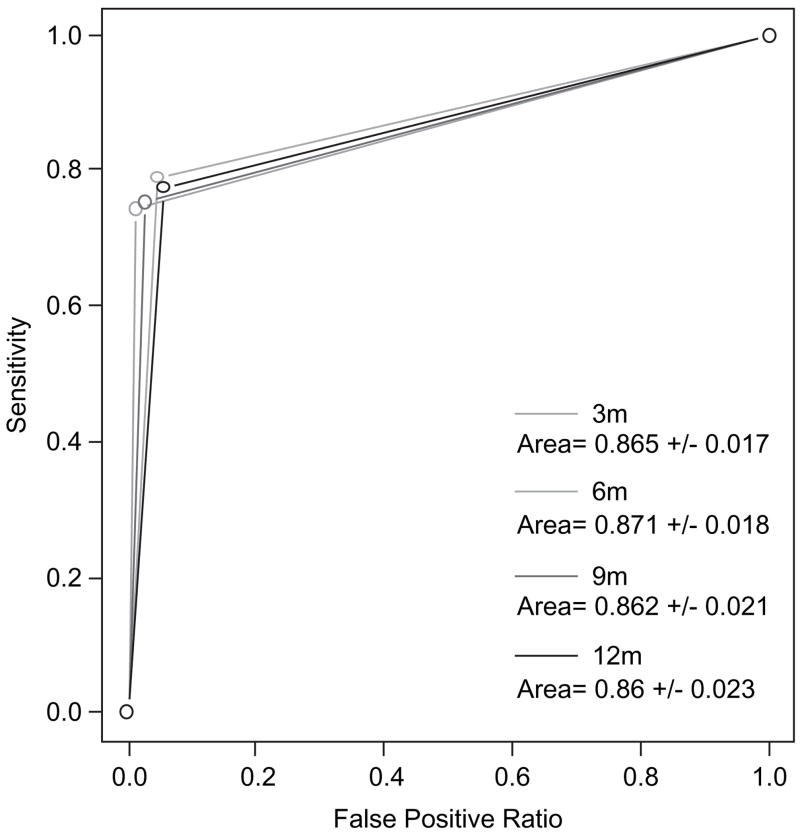
- Strong ROC Curve Areas: ROC curve analysis, which visually represents the trade-off between sensitivity and specificity, showed areas under the curve consistently above 0.86 for the Mannheimer tool in predicting three-day self-reported adherence. Areas closer to 1.0 indicate excellent predictive accuracy.
These findings confirm that the Mannheimer AIDS Care Adherence Screening Tool provides a valid and reliable measure of self-reported adherence, comparable to the more time-consuming three-day self-report.
Caption: Receiver Operating Characteristic (ROC) curves comparing the Mannheimer AIDS Care Adherence Screening Tool and the three-day self-report in predicting viral suppression at 12 months. The Mannheimer tool shows comparable, and in some cases superior, predictive power.
Prediction of Virologic Outcomes (HIV RNA)
The study further assessed the ability of the Mannheimer AIDS Care Adherence Screening Tool to predict virologic outcomes, specifically HIV RNA suppression. The results showed:
- Significant Association with Viral Suppression: Participants with Mannheimer Adherence Index scores >10 were significantly more likely to achieve a 1-log decrease in HIV RNA and to have undetectable HIV RNA levels compared to those with scores ≤10.
- Comparable or Superior to Three-Day Self-Report: While both measures were associated with virologic outcomes, the Mannheimer tool demonstrated a consistently stronger association, with higher odds ratios for viral suppression across multiple time points. ROC curve analysis also suggested a more robust relationship between the Mannheimer tool and virologic outcome, particularly at 12 months, where the difference in ROC areas was statistically significant.
These findings indicate that the Mannheimer AIDS Care Adherence Screening Tool is not only a valid measure of self-reported adherence but also a clinically meaningful predictor of virologic response to ART.
Impact on Immunologic Outcomes (CD4 Counts)
The study also examined the relationship between adherence measures and changes in CD4 counts, a marker of immune function. The analysis revealed:
- Significant Association with CD4 Count Increase at 12 Months: Participants with baseline Mannheimer Adherence Index scores >10 experienced a significantly greater mean increase in CD4 cell count over 12 months (98 cells) compared to those with scores ≤10 (41 cells). This difference was statistically significant.
- Less Consistent Association at Earlier Time Points: While a trend towards greater CD4 count increases was observed in the adherent group as defined by the Mannheimer tool at earlier time points (3, 6, and 9 months), these differences were not statistically significant. The three-day self-report did not show a significant association with CD4 count changes at any time point.
These results suggest that the Mannheimer AIDS Care Adherence Screening Tool is a useful predictor of longer-term immunologic recovery in individuals on ART, demonstrating its clinical relevance beyond virologic outcomes.
Discussion: Implications and Advantages of the Mannheimer Tool
The findings of this study underscore the value of the Mannheimer AIDS Care Adherence Screening Tool as a practical and effective measure of ART adherence. Its key advantages and implications for clinical practice are discussed below.
Practical Applications in Clinical Settings
The Mannheimer AIDS Care Adherence Screening Tool offers several practical advantages for routine clinical use:
- Ease of Administration: The three-question format is quick and easy to administer during routine clinic visits, minimizing disruption to workflow.
- Simplicity of Scoring: The straightforward scoring system allows for immediate calculation of the adherence index, providing clinicians with rapid feedback.
- Reduced Patient Burden: The brevity of the tool reduces the burden on patients compared to more lengthy adherence questionnaires.
- Utility in Diverse Settings: Validated in diverse clinical settings and populations, the tool is applicable across a wide range of HIV care contexts.
- Potential for Improved Adherence Support: By providing a quick and reliable adherence assessment, the tool can facilitate timely identification of adherence challenges and enable targeted interventions to support patients in maintaining optimal ART adherence.
The Mannheimer AIDS Care Adherence Screening Tool can be easily integrated into routine HIV care, empowering healthcare providers to monitor adherence effectively and proactively address adherence barriers.
Strengths and Limitations
This study has several strengths, including its large sample size, multi-site design, diverse patient population, and robust validation methodology. The comparison to the established three-day self-report and the examination of clinical outcomes strengthen the validity of the findings. However, some limitations should be considered:
- Self-Report Bias: Like all self-report measures, the Mannheimer tool is susceptible to social desirability bias, where patients may overestimate their adherence. However, the study suggests that the more general nature of the Mannheimer questions may potentially mitigate this bias compared to more direct recall methods.
- Study Attrition: Participant attrition over the 12-month study period may introduce some bias. However, the analyses were conducted on available data at each time point, and the remaining sample still represented a diverse group reflecting the US HIV epidemic.
- Focus on Antiretroviral-Experienced Individuals: The study population consisted of ART-experienced individuals. Further research may be needed to validate the tool in ART-naïve populations.
Despite these limitations, the evidence strongly supports the reliability and validity of the Mannheimer AIDS Care Adherence Screening Tool as a valuable tool for assessing ART adherence.
Future Research Directions
Future research could further explore the utility of the Mannheimer AIDS Care Adherence Screening Tool in various contexts:
- Validation in ART-Naïve Populations: Investigating its performance in individuals initiating ART for the first time.
- Comparison to Objective Measures: Comparing the tool to more objective adherence measures such as electronic drug monitoring to further assess its accuracy.
- Impact on Clinical Practice: Evaluating the impact of routine use of the Mannheimer tool on adherence rates, virologic outcomes, and patient engagement in care.
- Cultural Adaptations: Exploring the need for and feasibility of cultural adaptations of the tool for diverse populations and languages.
Further research will continue to refine our understanding of the Mannheimer AIDS Care Adherence Screening Tool and maximize its potential to improve HIV care.
Conclusion: The Mannheimer AIDS Care Adherence Screening Tool as a Valuable Asset
The Mannheimer AIDS Care Adherence Screening Tool, or CASE Adherence Index, stands out as a valuable asset in the arsenal of tools for HIV care. Its brevity, simplicity, and demonstrated validity make it highly suitable for routine clinical use. This study provides compelling evidence that the Mannheimer tool is:
- A Valid Measure of Self-Reported Adherence: Strongly correlated with the established three-day self-report.
- A Clinically Meaningful Predictor: Significantly associated with virologic suppression and longer-term immunologic recovery.
- A Practical and Efficient Tool: Easy to administer, score, and interpret, making it ideal for busy clinical settings.
By incorporating the Mannheimer AIDS Care Adherence Screening Tool into routine HIV care, healthcare providers can enhance their ability to monitor ART adherence, identify patients needing additional support, and ultimately improve treatment outcomes for people living with HIV. This simple yet powerful tool has the potential to make a significant contribution to optimizing HIV care and improving the lives of individuals on ART.